Hepatic arterial infusion chemotherapy combined with bevacizumab plus a PD-1 inhibitor for gallbladder cancer with hepatic oligometastasis: a real-world study
Highlight box
Key findings
• The combination of hepatic artery infusion chemotherapy (HAIC), programmed death receptor-1 (PD-1) inhibitor and bevacizumab showed its potential value for advanced gallbladder cancer (GBC) with hepatic oligometastasis.
What is known and what is new?
• The current first-line choice for advanced GBC is gemcitabine plus cisplatin combined with durvalumab.
• The strategy of HAIC (oxaliplatin, 5-fluorouracil, leucovorin) combined with bevacizumab plus PD-1 inhibitor has promising prospect in treating GBC with hepatic oligometastasis. The safety and tolerance were acceptable.
What is the implication, and what should change now?
• Locoregional chemotherapy combined with immune-targeted therapy should be considered reasonably for selected patients with advanced GBC.
Introduction
Gallbladder cancer (GBC) accounts for 80–95% of all biliary tract cancers (BTCs) (1). The prognosis for GBC is extremely poor with median overall survival (OS) of 4–7 months (2). Radical resection is the first option and only curative treatment for GBC. In patients who receive a curative resection, 5-year OS rates range from 15% to 20% (3). Unfortunately, most GBC cases are diagnosed at an advanced or terminal stage and are contraindicated for surgery (4). Patients with unresectable GBC have a dismal prognosis with 5-year OS rates of <5% (5).
The current first-line choice of chemotherapy for advanced BTC is gemcitabine plus cisplatin (GC). Recently, two randomized, double-blind phase III trials demonstrated that GC plus immunotherapy shows promising efficacy and acceptable safety in patients with advanced BTC with median OS of 12.7–12.8 months (6,7). In accordance with the National Comprehensive Cancer Network (NCCN) guideline, GC plus durvalumab, a programmed cell death ligand 1 (PD-L1) inhibitor, is considered to be the preferred regimen for unresectable BTC (8). Several new strategies have been evaluated for BTC treatment. Targeted-immune therapy offers another option and exerts a synergistic effect with chemotherapy. A programmed death receptor-1 (PD-1) inhibitor plus lenvatinib for advanced GBC achieved an objective response rate (ORR) of 32.3% (9). Hepatic artery infusion chemotherapy (HAIC) has achieved encouraging response rates in hepatocellular carcinoma (HCC) and colorectal liver metastasis (10,11). HAIC has been recently proven to be effective for patients with intrahepatic and perihilar cholangiocarcinoma (12,13). In patients with GBC, HAIC with oxaliplatin and 5-fluorouracil achieved an ORR of 69.2% (14). Based on results above, for well-tolerated patients, the combination of multiple treatment modalities is reasonable and beneficial.
GBC is quite different from other BTCs in terms of molecular phenotype, microenvironment, and survival outcome. The data of GBC in clinical trials are often included in the basket of BTC. Specific treatments for GBC should be urgently explored. GBC frequently spreads to the liver (15). The term oligometastasis indicates that tumors progress to a limited number of metastatic lesions with the potential to benefit from local therapies, such as ablation, resection, and stereotactic radiotherapy (16). Cases of hepatic oligometastasis without hilar invasion and distant lymphatic metastasis account for a small proportion of GBC patients. However, these patients usually have adequate liver functions and limited tumor involvement, and have the potential to be converted to resection. Long-term survival has been reported in this group of patients (17,18). Therefore, GBC patients with hepatic oligometastasis may benefit from multidisciplinary treatments (MDTs).
Owing to the promising results, we preliminary explored the safety and effect of HAIC (oxaliplatin, 5-fluorouracil, and leucovorin, FOLFOX) combined with bevacizumab plus a PD-1 inhibitor for GBC with hepatic oligometastasis. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-816/rc).
Methods
Study population
From January 2020 to December 2022, all GBC patients who received treatment at the Branch Ward of the Faculty of Hepato-Pancreato-Biliary Surgery, Chinese People’s Liberation Army (PLA) General Hospital were reviewed. The inclusion criteria were as follows: diagnosed as GBC by pathological examination; hepatic metastasis, defined as a discrete hepatic lesion separate from the primary tumor, measured by computed tomography (CT) or magnetic resonance imaging (MRI); radiographically detectable lymph node metastasis was confined to the hilar of liver (lymph metastasis was considered if any of the following criteria were met: short-axis diameter larger than 10 mm, abnormal round morphology, non-uniform density, non-uniform enhancement, internal necrosis, lymph node fusion, ill-defined borders, or involvement of surrounding organs or blood vessels) (19); received HAIC + bevacizumab + a PD-1 inhibitor as first therapy; and adequate liver function (Child-Pugh A) and other vital organ functions before treatment. The exclusion criteria were as follows: GBC with obstructive jaundice; GBC with invasion of the hepatic artery or portal vein in the hilar region; and previous other forms of treatment, such as resection, systematic chemotherapy, or radiotherapy. This retrospective study was approved by the Ethics Committee of the Sixth Medical Center of Chinese PLA General Hospital (No. HZKY-PJ-2023-44). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All treatment decisions were made at the patient’s discretion with informed consent. Patient privacy was fully protected.
Pretreatment assessment
Abdominal ultrasonography, enhanced CT, or MRI was used to assess the size and location of GBC and hepatic metastasis. Thoracic CT was performed to evaluate signs of lung metastasis. 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) was used to detect distal metastasis. The maximum 18F-FDG standard uptake value (SUV) in the delay phase was collected in this study. Tumor markers, including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), were routinely examined.
A complete blood count was obtained for each patient. Liver function was evaluated by measuring serum total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin, albumin (ALB), prealbumin, alanine transaminase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transpeptidase (GGT) levels. Prothrombin time (PT) was measured to evaluate liver function and surgical safety. Routine pulmonary function tests and cardiovascular Doppler ultrasound were performed to evaluate signs of any contraindications to resection.
Treatment
Bevacizumab [Avastin, Roche Pharma (Switzerland) Ltd.] was administered at 7.5 mg/kg every 3 weeks until tumor progression or the occurrence of intolerant adverse events (AEs). Sintilimab, a PD-1 inhibitor (Innovent Biologics Suzhou Co. Ltd., China), was administered at 200 mg every 3 weeks, starting on the same day of bevacizumab administration.
HAIC was performed within 3 days after sintilimab and bevacizumab were administrated. After femoral artery puncture and catheterization, arteriography of the coeliac artery (CA) and superior mesenteric artery (SMA) was performed to detect the tumor blood supply. Then, a microcatheter (Terumo Corp., Tokyo, Japan) was inserted into the proper hepatic artery. We did not embolize gastroduodenal artery (GDA) and right gastric artery (RGA) which are routinely performed at other centers (10,12,14) to make the chemotherapeutics cover the hilar region of the liver. A modified FOLFOX-6 regimen (oxaliplatin at 85 mg/m2 from hour 0 to 2 on day 1; leucovorin at 400 mg/m2 from hour 2 to 3 on day 1; 5-fluorouracil at 400 mg/m2 bolus at hour 3 on day 1; 5-fluorouracil at 2,400 mg/m2 over 46 hours on days 1–2) was then administrated.
Evaluation of the tumor response
The combination therapy was administered in a 3-week cycle. After every two cycles, the tumor response was evaluated by RECIST 1.1 (response evaluation criteria in solid tumor 1.1) by radiologists at the Department of Medical Imaging (20). The clinical outcomes were defined as a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The ORR was calculated as the proportion of cases with a best overall response of CR or PR. The disease control rate (DCR) was calculated as the proportion of cases with a best overall response of CR, PR, or SD. If the following conditions were met, surgical resection was considered: tumors were significantly reduced in size without extrahepatic metastasis; no new lesions detected; and tumors were evaluated to be safe to resect. For patients with SD or PD, systematic therapy continued depending on the patient’s tolerance. Once extrahepatic metastasis was observed, systematic chemotherapy was applied instead of HAIC. The maximum course of HAIC treatment did not exceed six cycles. All recommendations were made by a MDT conference, including specialists in oncology, surgery, radiology, and interventional therapy. The final decisions were made at the patient’s discretion with informed consent. AEs were assessed by CTCAE (Common Terminology Criteria for Adverse Events, version 5.0).
Follow-up
All patients were closely followed up by outpatient visits or social network platforms because of the coronavirus pandemic. Laboratory examinations were performed every 2–4 weeks, and CT/MRI was performed regularly. 18F-FDG PET-CT was performed every two to four cycles.
Statistical analysis
Continuous data are expressed as the mean ± standard deviation and were compared using the unpaired t-test. When continuous variable data did not conform to a normal distribution, data are expressed as the median (range) and were compared using the Mann-Whitney U-test. Categorical data were compared by the χ2 test with Fisher’s exact test. Survival analyses were performed by the Kaplan-Meier method. OS was calculated from the initial day of combination treatment to the day of death or the most recent follow-up visit. Progression-free survival (PFS) was calculated from the initial day of combination treatment to the first follow-up visit at which clear evidence of tumor progression was observed or the most recent follow-up visit. A P value of less than 0.05 was considered significant. All statistical analyses were performed using IBM SPSS for Windows, version 23.0 (IBM Corp., Armonk, NY, USA) and R program (Version 4.2.2).
Results
Baseline characteristics of enrolled patients
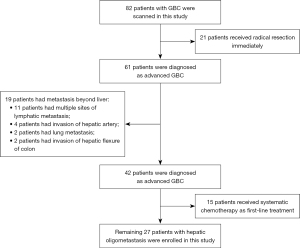
A total of 82 GBC patients who received treatment at our center were assessed. Fifty-five patients were excluded from the study population. Among them, 21 patients received an upfront radical resection, 19 patients had extrahepatic metastasis, and 15 patients received systematic chemotherapy. The remaining 27 patients who received HAIC combined with bevacizumab plus sintilimab were enrolled in this study (Figure 1). Their baseline characteristics are shown in Table 1.
Table 1
| Variables | Total (n=27) |
|---|---|
| Age (years) | 66 [45–85] |
| Gender | |
| Female | 15 (55.6) |
| Male | 12 (44.4) |
| ECOG performance score | |
| 0 | 25 (92.6) |
| 1 | 2 (7.4) |
| CA19-9 | |
| >1,900 U/mL | 18 (66.7) |
| ≤1,900 U/mL | 9 (33.3) |
| Site of gallbladder cancer | |
| Peritoneal side | 0 |
| Hepatic side | 18 (66.7) |
| Diffused | 9 (33.3) |
| Number of liver metastasis | |
| 1–3 | 21 (77.8) |
| 4–5 | 3 (11.1) |
| >5 | 3 (11.1) |
| Detectable hilar lymph metastasis† | 9 (33.3) |
| Extent of metastasis involvement | |
| Limited to hemiliver | 17 (63.0) |
| Extended to hemiliver | 10 (37.0) |
| Size of primary lesions (cm) | 3.5 [1.9–5.2] |
| Largest size of metastasis (cm) | 3.2 [1.0–10.1] |
| 18F-FDG standard uptake value‡ | |
| Primary gallbladder lesions | 5.3 [2.6–16.5] |
| Hepatic metastasis | 4.5 [3.1–15.1] |
| Preoperative laboratory test | |
| WBC (×109/L) | 6.91 [3.11–10.51] |
| HGB (g/L) | 119 [86–144] |
| PLT (×109/L) | 246 [135–357] |
| PT (s) | 11.9 [11.0–13.8] |
| TBIL (µmol/L) | 11.2 [6.8–40.1] |
| ALB (g/L) | 35.9 [31.1–46.1] |
| ALT (U/mL) | 30.8 [10.7–111.7] |
| GGT (U/mL) | 99.8 [22.9–662.9] |
| Creatinine (mmol/L) | 69.3 [49.8–98.1] |
Data are presented as n (%) or median [range]. †, CT-determined lymph metastasis, which was considered if any of the following criteria were met: short-axis diameter larger than 10 mm, abnormal round morphology, non-uniform density, non-uniform enhancement, internal necrosis, LN fusion, ill-defined borders, or involvement of surrounding organs or blood vessels (18); ‡, 18F-FDG standard uptake value was the maximum value of lesions in delay phase. For hepatic metastasis, we adopt the value of target lesions. ECOG, eastern cooperative oncology group; CA19-9, carbohydrate antigen 19-9; 18F-FDG, 18F-fluorodeoxyglucose; WBC, white blood cells; HGB, hemoglobulin; PLT, platelet; PT, prothrombin time; TBIL, total bilirubin; ALB, albumin; ALT, alanine aminotransferase; GGT, gamma-glutamine transpeptidase; CT, computed tomography.
Notably, 18 (66.7%) patients had CA19-9 >1,900 U/mL (the upper limit of the measurable value in our center). All patients had a primary lesion on the hepatic side of the gallbladder. Six (22.2%) patients had more than three lesions of hepatic metastasis. Ten (37.0%) patients had liver metastasis spreading over the hemiliver. Nine patients (33.3%) had radiographically detectable lymph metastasis at the hilar of liver. The median size of primary lesions was 3.5 cm (range, 1.9–5.2 cm). The median size of metastatic lesions was 3.2 cm (range, 1.0–10.1 cm).
Tumor response and short-term survival
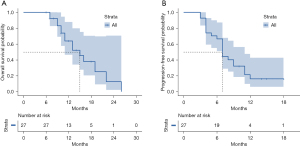
For all patients enrolled in this study, the median time of follow-up was 11.0 months (range, 6–26 months). In brief, no patient achieved CR, 15 patients achieved PR, eight patients exhibited SD, and four patients exhibited PD. The overall ORR was 55.6% and DCR was 85.2%. For all patients, the median OS was 15.0 months [range, 11–not applicable (NA) months], and the 1-year survival rate was 64.0% (Figure 2A). Median PFS was 7.0 months (range, 7–11 months), and the 1-year PFS rate was 16.2% (Figure 2B).
We further compared the treatment response of primary gallbladder lesions and hepatic metastasis. For primary gallbladder lesions, one patient achieved CR, 20 patients achieved PR, and six patients exhibited SD. The ORR of primary lesions was 77.8%, and the DCR was 100%. For hepatic metastasis, no patient achieved CR, 11 patients achieved PR, 13 patients exhibited SD, and three patients exhibited PD (Figure 3). Compared with hepatic metastasis, primary lesions were more likely to exhibit remission after combination therapy (Table 2, ORR: 77.8% vs. 40.7%, P=0.012).
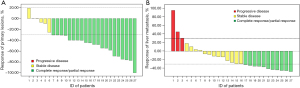
Table 2
| Tumor response | Overall response | Response | ||
|---|---|---|---|---|
| Primary lesions | Hepatic metastasis | P value | ||
| Complete response | 0 | 1 | 0 | 0.027 |
| Partial response | 15 | 20 | 11 | – |
| Stable disease | 8 | 6 | 13 | – |
| Progressive diseases | 4 | 0 | 3 | – |
| Objective response rate | 55.6% | 77.8% | 40.7% | 0.012 |
| Disease control rate | 85.2% | 100% | 88.9% | 0.236 |
RECIST 1.1, response evaluation criteria in solid tumor 1.1.
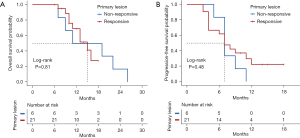
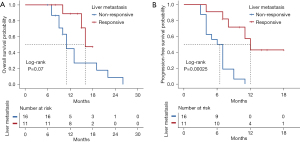
We also compared the effect of response on prognosis between primary lesions and hepatic metastasis. When we focused on primary lesions, median OS was 15.0 months (range, 11–NA months) for patients who achieved a response (CR or PR) compared with 15.0 months (range, 9–NA months) for patients who exhibited a non-response (SD or PD) (P=0.809, Figure 4A). Median PFS was 7 months (range, 4–NA months) for patients who achieved a response and 7 months (range, 7–NA months) for patients who exhibited a non-response (P=0.447, Figure 4B). When we focused on hepatic metastasis, median OS was 16 months (range, 15–NA months) for patients who achieved a response compared with 11 months (range, 10–NA months) for patients who exhibited a non-response (P=0.070, Figure 5A). Median PFS was 12 months (range, 8–NA months) for patients who achieved a response compared with 6.5 months (range, 4–9 months) for who patients exhibited a non-response (P<0.001, Figure 5B). These results suggest that remission of liver metastasis appears to be more beneficial for long-term survival.
Successful conversion to resection
Six patients in our cohort were successfully converted to resection (22.2%). Cholecystectomy plus regional lymphadenectomy (groups 8, 12, 13a, and 16a2) was routinely performed. Among the patients, two received a right hepatectomy, one received a left hepatectomy plus partial resection of segment 5, one received a resection of segments 5 and 4b, and two received a wedge resection of the gallbladder bed and a local resection of hepatic lesions. The characteristics are shown in Table 3. A representative case is shown in Figure 6.
Table 3
| Characteristics | Values |
|---|---|
| Tumor response | |
| Partial response | 4 (66.7) |
| Stable disease | 2 (33.3) |
| Cycles of combination therapy | |
| 2 | 3 (50.0) |
| 3 | 3 (50.0) |
| Postoperative adjuvant chemotherapy | |
| Gemcitabine + albumin-bound paclitaxel | 1 (16.7) |
| Gemcitabine + S1 (tegafur gimeracil oteracil potassium capsule) | 2 (33.3) |
| S1 (tegafur gimeracil oteracil potassium capsule) | 3 (50.0) |
| Type of hepatic resection | |
| Right hemihepatectomy | 2 (33.3) |
| Left hepatectomy plus partial resection of segment 5 | 1 (16.7) |
| Segment 5 and 4b | 1 (16.7) |
| Wedge resection of gallbladder bed and local resection of hepatic lesions | 2 (33.3) |
| Surgical complications | |
| Pleural effusion | 6 (100.0) |
| Classification complications | |
| Clavien-Dindo I | 5 (83.3) |
| Clavien-Dindo IIIa | 1 (16.7) |
Data are presented as n (%).

Safety
The median number of cycles of combination therapy was four (range, two to six cycles). No patient died during the treatment. No grade 4 AEs were observed. All AEs that occurred during combination therapy are summarized in Table 4. The main AEs were blood disorders such as neutropenia (92.6%), anemia (44.4%), and thrombocytopenia (55.6%). Other AEs included hypertension (7.4%), hypothyroidism (14.8%), diarrhea (25.9%), vomiting (63.0%), decreased appetite (44.4%), bilirubin elevation (11.1%), ALT elevation (7.4%), and dysesthesia (3.7%). Only six patients experienced grade 3 neutropenia (22.2%). All AEs were alleviated after management.
Table 4
| Adverse events | Total (n=27) | Grade 1–2 | Grade 3 |
|---|---|---|---|
| Blood and lymphatic disorders | |||
| Neutropenia | 25 (92.6) | 19 (70.4) | 6 (22.2) |
| Anemia | 12 (44.4) | 12 (44.4) | 0 |
| Thrombocytopenia | 15 (55.6) | 15 (55.6) | 0 |
| Cardiac disorders | |||
| Hypertension | 2 (7.4) | 2 (7.4) | 0 |
| Endocrine disorders | |||
| Hypothyroidism | 4 (14.8) | 4 (14.8) | 0 |
| Gastrointestinal disorders | |||
| Vomiting | 17 (63.0) | 17 (63.0) | 0 |
| Diarrhea | 7 (25.9) | 7 (25.9) | 0 |
| Decreased appetite | 12 (44.4) | 12 (44.4) | 0 |
| Hepatobiliary disorders | |||
| Bilirubin elevation | 3 (11.1) | 3 (11.1) | 0 |
| ALT elevation | 2 (7.4) | 2 (7.4) | 0 |
| Nervous system disorders | |||
| Dysesthesia | 1 (3.7) | 1 (3.7) | 0 |
Data are presented as n (%). ALT, alanine aminotransferase.
Subgroup analysis in accordance with the response of hepatic metastasis
Among the patients, 11 (40.7%) patients with hepatic metastasis achieved CR or PR after combination therapy. We preliminarily compared the clinical characteristics between patients who achieved a response (CR or PR) and those who exhibited a non-response (SD or PD) (Table 5). The results showed that the gallbladder lesion site, and number and distribution of metastatic lesion were not significantly associated with the response after combination treatment. The preoperative level of CA19-9 (>1,900 U/mL, 36.4% vs. 81.3%, P=0.040) and the size of the largest lesion (>5 cm, 27.3% vs. 75.0%, P=0.022) were more common in SD and PD groups. A significant decrease in the 18F-FDG SUV value in PET-CT scans was more common in CR and PR groups (decrease >50%, 81.2% vs. 18.8%, P=0.002; decrease >90%, 63.6% vs. 0.0%, P<0.001).
Table 5
| Clinical data | Hepatic metastasis | P value | |
|---|---|---|---|
| Achieved response (n=11) | Without response (n=16) | ||
| Age >60 years | 8 (72.7) | 12 (75.0) | >0.999 |
| Gender | 0.448 | ||
| Female | 3 (27.3) | 7 (43.8) | |
| Male | 8 (72.7) | 9 (56.3) | |
| ECOG score | >0.999 | ||
| 0 | 10 (90.9) | 15 (93.8) | |
| 1 | 1 (9.1) | 1 (6.3) | |
| CA19-9 | 0.040 | ||
| >1,900 U/mL | 4 (36.4) | 13 (81.3) | |
| CA19-9 decrease† | |||
| >90% | 7 (70.0) | 6 (37.5) | 0.226 |
| Site of gallbladder cancer | 0.692 | ||
| Peritoneal side | 0 | 0 | |
| Hepatic side | 8 (72.7) | 10 (62.5) | |
| Diffused | 3 (27.3) | 6 (37.5) | |
| Number of liver metastasis | >0.999 | ||
| 1–3 | 9 (81.8) | 12 (75.0) | |
| >3 | 2 (18.2) | 4 (25.0) | |
| Metastasis involvement | >0.999 | ||
| Limited to hemiliver | 7 (63.6) | 10 (62.5) | |
| Extended to hemiliver | 4 (36.4) | 6 (37.5) | |
| Largest size of metastasis >5 cm | 3 (27.3) | 12 (75.0) | 0.022 |
| 18F-FDG SUVmax decrease‡ | |||
| >50% | 9 (81.2) | 3 (18.8) | 0.002 |
| >90% | 7 (63.6) | 0 | <0.001 |
| Response of metastatic lymph nodes | n=5 | n=4 | – |
| PR | 4 (80.0) | 2 (50.0) | |
| SD | 1 (20.0) | 1 (25.0) | |
| PD | 0 | 1 (25.0) | |
| Preoperative laboratory test | |||
| WBC (×109/L) | 7.8 [3.9–10.5] | 6.39 [3.1–8.1] | 0.110 |
| HGB (g/L) | 109 [90–128] | 122 [84–140] | 0.054 |
| PLT (×109/L) | 271 [245–339] | 227.5 [135–315] | 0.054 |
| PT (s) | 12.1 [11.1–13.4] | 11.9 [10.2–13.8] | 0.512 |
| TBIL (µmol/L) | 10.6 [6.8–26.9] | 11.7 [6.8–40.1] | 0.790 |
| ALB (g/L) | 34.9 [32.3–39.9] | 37.0 [31.1–41.1] | 0.110 |
| ALT (U/mL) | 53.0 [10.7–111.7] | 30.4 [13.3–99.3] | 0.577 |
| GGT (U/mL) | 90.1 [65.1–1,932.0] | 110.2 [31.3–662.9] | 0.942 |
| Creatinine (mmol/L) | 62.0 [51.4–92.0] | 70.5 [57.8–98.1] | 0.440 |
Data are presented as n (%), n or median [range]. †, there was one patient with no elevation in CA19-9 level who was excluded from this analysis; ‡, the 18F-FDG SUVmax value was the maximum value of lesion in delay phase during PET-CT scan. ECOG, eastern cooperative oncology group; CA19-9, carbohydrate antigen 19-9; 18F-FDG, 18F-fluorodeoxyglucose; PR, partial response; SD, stable disease; PD, progressive disease; WBC, white blood cells; HGB, hemoglobulin; PLT, platelet; PT, prothrombin time; TBIL, total bilirubin; ALB, albumin; ALT, alanine aminotransferase; GGT, gamma-glutamine transpeptidase; SUV, standard uptake value; PET, positron emission tomography; CT, computed tomography.
Discussion
In addition to its anatomical position, GBC is quite different from other BTCs in terms of clinicopathology and molecular insights. Treatments that specifically target GBC remain limited (21). In the present study, we preliminarily investigated the effect of HAIC (FOLFOX regimen) combined with bevacizumab plus a PD-1 inhibitor for treatment of GBC with hepatic oligometastasis. This is an exploratory study in this subgroup of GBC patients. Most patients in this cohort had >1,900 U/mL CA19-9 (66.7%), 22.2% of patients had more than three lesions of hepatic metastasis, and 37.0% patients had liver metastasis spreading over the hemiliver, implying poor prognoses. After combination therapy, they achieved an ORR of 55.6% and a DCR of 85.2%. Median OS was 15.0 months and median PFS was 7.0 months. Six patients (22.2%) were successfully converted to resection. Compared with primary lesions, patients with hepatic metastasis appeared more difficult to achieve remission after the combination therapy (ORR: 40.7% vs. 77.8%; P=0.012), but their response appeared to be closely related to prognosis (median OS: 16.0 months in CR and PR groups vs. 11.0 months in SD and PD groups, P=0.070; median PFS: 12.0 months in CR and PR groups vs. 6.5 months in SD and PD groups, P<0.001). Moreover, the safety and tolerance were acceptable. These results suggest that this strategy has promising prospects.
In this study, hepatic metastasis was defined as a discrete hepatic lesion separate from the primary tumor. Advanced GBC has a tendency to invade the hepatic hilum or hepatoduodenal ligament and often displays lymphatic metastasis. GBC cases with hepatic oligometastasis account for a small proportion of the whole GBC population. These discrete lesions are likely to be of hematogenous origin. Metastatic nodules that spread through a hematogenous route have a poor outcome after resection, irrespective of the type of hepatectomy (22). Furthermore, some of these patients tend to have slightly impaired liver functions without obstructive jaundice and limited tumor involvement without extrahepatic metastasis. Hence, they have not reached uncontrolled systemic metastasis and have the opportunity to be converted to resection after effective treatment. Although they are not the major proportion of unresectable GBC patients, it is reasonable to investigate new strategies because there are limited treatment options at present.
The current frontline chemotherapy for unresectable BTCs including GBC is GC (8). However, the benefit remains poor with an ORR of 21–37% (23). In recent years, HAIC has been a focus as a locoregional chemotherapy. During the HAIC procedure, chemotherapeutic agents are injected directly and continuously into the liver via the hepatic artery. High concentrations of these agents at the tumor site are expected to increase antitumor effects. The first-pass effect results in high local drug concentrations in the liver with minimal systemic distribution, which significantly reduces systemic AEs (24). For GBC with hepatic oligometastasis, HAIC has several potential advantages. Lesions of liver metastasis mainly receive an arterial blood supply (25). Because the cystic artery arises commonly from the right hepatic artery, chemotherapy agents can be administrated to primary gallbladder lesions and hepatic metastasis simultaneously. BTCs are also dense, desmoplastic tumors characterized by a poorly immunogenic tumor microenvironment. These factors contribute to the resistance of GBC to chemotherapy (26). Continuous infusion of chemotherapeutics prolongs contact time and increases the local concentration, which improve the effect. Different from other locoregional treatments, such as radiofrequency ablation (RFA), transarterial chemoembolization (TACE), and radiotherapy, locoregional chemotherapy is also effective for invisible micrometastasis. At present, no studies have reported HAIC therapy using the GC regimen. However, FOLFOX is recommended for neoadjuvant therapy and is a second-line regimen for unresectable and metastatic BTC (27). The FOLFOX protocol has been used for HAIC treatment for a long time, and its safety and efficacy have been confirmed in HCC patients (10). Zheng et al. investigated HAIC with oxaliplatin and 5-fluorouracil for treatment of advanced GBC. Their study enrolled 26 patients with advanced GBC and found that HAIC was well tolerated and achieved an ORR of 69.2% (14). Therefore, we used FOLFOX for HAIC in this study. Hilar lymph nodes are usually the first site of lymph node metastasis in GBC patients. In theory, the blood supply of hilar lymph nodes comes from the capillary branch of the proper hepatic artery. During the HAIC procedure, we did not embolize the GDA or RGA, which is routinely performed at other centers (10,12,14). This strategy makes chemotherapeutics cover the area around the portal of the liver as much as possible.
Targeted therapy based on specific molecular aberrations, such as fibroblast growth factor receptors-2 (FGFR-2) fusions, isocitrate dehydrogenase-1 (IDH-1) mutations, and human epidermal growth factor-2 (HER-2) amplification or overexpression, has been reported to achieve survival improvement (28). However, these molecular aberrations only occur in a small proportion of GBC patients (26). Anti-angiogenesis therapy is another important field of targeted therapy. Vascular endothelial growth factor (VEGF), the primary growth factor regulating angiogenesis, is over-expressed in 45–75% of BTCs and has been implicated in the control of lymphangiogenesis and lymphatic metastasis in GBC patients (29). In the setting of targeted immunotherapy, VEGF-driven angiogenesis is a logical target. Tyrosine kinase inhibitors (TKIs; e.g., lenvatinib) and monoclonal antibodies (e.g., bevacizumab) are the main choice of anti-angiogenic drugs. Several studies have reported the efficacy and safety of a PD-1 inhibitor plus lenvatinib for advanced GBC with an ORR of 32.3% (9,30). In fact, bevacizumab is more widely used. In the setting of targeted immunotherapy, bevacizumab combined with a PD-1/PD-L1 inhibitor have achieved encouraging results in HCC patients (31). Notably, bevacizumab combined with a PD-1/PD-L1 inhibitor has achieved anti-tumor efficacy in hepatocellular-cholangiocarcinoma (32), which implies effectiveness against BTC. Case reports have shown their potential value for GBC treatment (18,33). Sintilimab is a PD-1 inhibitor, which is often used in China. Bevacizumab plus sintilimab have achieved encouraging results in the treatment of HCC (34). Hence, we evaluated bevacizumab combined with sintilimab as a targeted immunotherapy in this study.
Synergies between targeted therapy, immunotherapy, and chemotherapy have been a research focus. Gemcitabine and cisplatin plus immunotherapy show promising efficacy (ORR: 26.7–29%, median OS: 12.7–12.8 months) and acceptable safety in patients with advanced BTC (6,7). In a single arm study, the combination of toripalimab (PD-1 inhibitor), lenvatinib, gemcitabine, and oxaliplatin achieved an encouraging ORR (80%) in advanced intrahepatic cholangiocarcinoma (ICC) patients and exhibited satisfactory safety (35). Therefore, we believe that HAIC combined with bevacizumab plus a PD-1 inhibitor has the potential for GBC treatment. No grade 4 AEs were observed in this study, and all AEs were alleviated after management. Mechanism of synergies between different treatment approaches have also been explored. Anti-VEGF targeted therapies enhance the effect of PD-1 inhibitor by reversing VEGF-mediated immunosuppression and promoting T-cell infiltration in tumors (11). Tumor cells are killed by locoregional chemotherapy and release more specific antigens which were captured by antigen-presenting cells and promote the effect of PD-1. Chemotherapeutic agents have also been shown to induce immunomodulatory effects (15,16). Six patients (22.2%) were successfully converted to resection. The only curative treatment for GBC is radical resection. On the topic of conversion therapy for GBC patients, the optimal treatment strategy remains controversial. Most studies have adopted treatments for advanced BTC or pancreatic cancer (21), and studies of GBC are limited. Our study provides potential new ideas for conversion therapy of unresectable GBC patients.
Compared with primary gallbladder lesions, we found that patients with hepatic metastasis appeared to have more difficulty achieving remission after the combination therapy, but their response appeared to be closely related to the prognosis. The size and number of hepatic metastases often exceed those of the primary lesion. Larger metastatic nodules make it difficult for drugs to enter the tumor, resulting in poor treatment efficacy. Further analysis of our cohort showed that large lesions of >5 cm were associated with a poor response of hepatic metastasis. A poor response of hepatic metastasis also indicated that tumors were refractory and more likely to spread through hepatic veins. Additionally, lesions of hepatic metastasis were more likely to invade the blood vessels and bile ducts in the liver hilum, resulting in deterioration of liver function and discontinuation of treatment.
Further comparison suggested that a preoperative level of >1,900 U/mL CA19-9 and the size of largest lesion being >5 cm were associated with an unsatisfactory response, whereas a significant decrease in 18F-FDG SUVmax was a marker of tumor remission. It is well established that the CA19-9 level is associated with tumor differentiation and strongly correlated to BTC prognosis (36). In our cohort, 66.7% of patients had >1,900 U/mL CA19-9 because all enrolled patients had a poor expected prognosis. A recent study indicated that high levels of 18F-FDG uptake are associated with poor differentiation and microvascular invasion in solid tumors (37). The findings from this study are consistent with the results of previous studies, indicating that a tumor-to-normal liver ratio of >2 (SUV-max of the tumor/SUV-mean of the normal liver) is associated with early tumor progression following PD-L1 inhibitor plus bevacizumab treatment (38). As a metabolic parameter, the significant decrease in 18F-FDG uptake following combination therapy is indicative of necrosis in tumors. Moreover, new extrahepatic metastasis can be detected by a whole-body PET-CT scan, which is crucial to evaluate the treatment response. Hence, in addition to CT/MRI-based evaluation criteria, 18F-FDG SUV and CA19-9 may be valuable biomarkers for accurate assessment of the treatment response.
This pilot study has several limitations. This is a retrospective study with a small sample size, and selective bias was inevitable. Additionally, multivariate analysis was not suitable because of the small sample size. Further research with larger sample sizes is needed to determine the long-term benefit of combination therapy. This study also did not compare the combination therapy with other potential strategies, such as chemotherapy or immune-targeted therapy. Moreover, only a small part proportion of patients with unresectable GBC can potentially benefit from this combination therapy.
Conclusions
HAIC combined with bevacizumab plus a PD-1 inhibitor has promising prospects for the treatment of GBC with hepatic oligometastasis. This study suggests that primary gallbladder lesions have a higher response rate, but the response of hepatic metastasis has more influence on the survival outcome. 18F-FDG uptake and CA19-9 have value as predictors of the treatment response. To validate the current findings, further research should include a randomized clinical trial to assess the safety and effectiveness of this treatment approach.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-816/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-816/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-816/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-816/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Ethics Committee of the Sixth Medical Center of Chinese PLA General Hospital (No. HZKY-PJ-2023-44). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All treatment decisions were made at the patient’s discretion with informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. Erratum in: CA Cancer J Clin 2020;70:313. [Crossref] [PubMed]
- Feo CF, Ginesu GC, Fancellu A, et al. Current management of incidental gallbladder cancer: A review. Int J Surg 2022;98:106234. [Crossref] [PubMed]
- Margonis GA, Gani F, Buettner S, et al. Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB (Oxford) 2016;18:872-8. [Crossref] [PubMed]
- Baiu I, Visser B. Gallbladder Cancer. JAMA 2018;320:1294. [Crossref] [PubMed]
- Zhou Y, Yuan K, Yang Y, et al. Gallbladder cancer: current and future treatment options. Front Pharmacol 2023;14:1183619. [Crossref] [PubMed]
- Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023;401:1853-65. [Crossref] [PubMed]
- Oh DY, Ruth He A, Qin S, et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid 2022;1:EVIDoa2200015.
- NCCN Clinical Practice Guidelines in Oncology. Hepatobiliary Cancers, Version 2.2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- Zuo B, Yang X, Yang X, et al. A real-world study of the efficacy and safety of anti-PD-1 antibodies plus lenvatinib in patients with advanced gallbladder cancer. Cancer Immunol Immunother 2022;71:1889-96. [Crossref] [PubMed]
- Li QJ, He MK, Chen HW, et al. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol 2022;40:150-60. [Crossref] [PubMed]
- Datta J, Narayan RR, Kemeny NE, et al. Role of Hepatic Artery Infusion Chemotherapy in Treatment of Initially Unresectable Colorectal Liver Metastases: A Review. JAMA Surg 2019;154:768-76. [Crossref] [PubMed]
- Wang X, Hu J, Cao G, et al. Phase II Study of Hepatic Arterial Infusion Chemotherapy with Oxaliplatin and 5-Fluorouracil for Advanced Perihilar Cholangiocarcinoma. Radiology 2017;283:580-9. [Crossref] [PubMed]
- Kasai K, Kooka Y, Suzuki Y, et al. Efficacy of hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic pegylated interferon α-2b for advanced intrahepatic cholangiocarcinoma. Ann Surg Oncol 2014;21:3638-45. [Crossref] [PubMed]
- Zheng K, Wang X, Cao G, et al. Hepatic Arterial Infusion Chemotherapy with Oxaliplatin and 5-Fluorouracil for Advanced Gallbladder Cancer. Cardiovasc Intervent Radiol 2021;44:271-80. [Crossref] [PubMed]
- Li Q, Li N, Gao Q, et al. The clinical impact of early recurrence and its recurrence patterns in patients with gallbladder carcinoma after radical resection. Eur J Surg Oncol 2023;49:106959. [Crossref] [PubMed]
- Ottaiano A, Santorsola M, Circelli L, et al. Oligo-Metastatic Cancers: Putative Biomarkers, Emerging Challenges and New Perspectives. Cancers (Basel) 2023;15:1827. [Crossref] [PubMed]
- Higuchi R, Ota T, Araida T, et al. Surgical approaches to advanced gallbladder cancer : a 40-year single-institution study of prognostic factors and resectability. Ann Surg Oncol 2014;21:4308-16. [Crossref] [PubMed]
- Ning C, Zhang X, Yang X, et al. Conversion therapy of stage IVb unresectable gallbladder carcinoma. Hepatobiliary Surg Nutr 2022;11:335-7. [Crossref] [PubMed]
- Zhan PC, Yang T, Zhang Y, et al. Radiomics using CT images for preoperative prediction of lymph node metastasis in perihilar cholangiocarcinoma: a multi-centric study. Eur Radiol 2023; Epub ahead of print. [Crossref]
- Schwartz LH, Seymour L, Litière S, et al. RECIST 1.1 - Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur J Cancer 2016;62:138-45. [Crossref] [PubMed]
- Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat Res Commun 2021;27:100354. [Crossref] [PubMed]
- Wakai T, Shirai Y, Sakata J, et al. Mode of hepatic spread from gallbladder carcinoma: an immunohistochemical analysis of 42 hepatectomized specimens. Am J Surg Pathol 2010;34:65-74. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Obi S, Sato S, Kawai T. Current Status of Hepatic Arterial Infusion Chemotherapy. Liver Cancer 2015;4:188-99. [Crossref] [PubMed]
- Lin G, Lunderquist A, Hägerstrand I, et al. Postmortem examination of the blood supply and vascular pattern of small liver metastases in man. Surgery 1984;96:517-26.
- Ilyas SI, Affo S, Goyal L, et al. Cholangiocarcinoma - novel biological insights and therapeutic strategies. Nat Rev Clin Oncol 2023;20:470-86. [Crossref] [PubMed]
- Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 2021;22:690-701. [Crossref] [PubMed]
- Javle M, Borad MJ, Azad NS, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 2021;22:1290-300. [Crossref] [PubMed]
- Lin W, Jiang L, Chen Y, et al. Vascular endothelial growth factor-D promotes growth, lymphangiogenesis and lymphatic metastasis in gallbladder cancer. Cancer Lett 2012;314:127-36. [Crossref] [PubMed]
- Lin J, Yang X, Long J, et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr 2020;9:414-24. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Gigante E, Bouattour M, Bedoya JU, et al. Atezolizumab and bevacizumab for non-resectable or metastatic combined hepatocellular-cholangiocarcinoma: A multicentric retrospective study. United European Gastroenterol J 2023; Epub ahead of print. [Crossref]
- Guo L, Zhang J, Liu X, et al. Successful Treatment of Metastatic Gallbladder Carcinoma with PD-L1 Expression by the Combination of PD-1 Inhibitor Plus Bevacizumab with Chemotherapy: A Case Report. Onco Targets Ther 2022;15:629-36. [Crossref] [PubMed]
- Zeng X, Jia Y, Chen H, et al. A real-world analysis of survival and cost-effectiveness of sintilimab plus bevacizumab biosimilar regimen in patients with advanced hepatocellular carcinoma. J Cancer Res Clin Oncol 2023;149:9213-9. [Crossref] [PubMed]
- Shi GM, Huang XY, Wu D, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther 2023;8:106. [Crossref] [PubMed]
- Lee DW, Im SA, Kim YJ, et al. CA19-9 or CEA Decline after the First Cycle of Treatment Predicts Survival in Advanced Biliary Tract Cancer Patients Treated with S-1 and Cisplatin Chemotherapy. Cancer Res Treat 2017;49:807-15. [Crossref] [PubMed]
- Uchida Y, Yoh T, Fukui A, et al. Complete Metabolic Response by 18 F-FDG PET/CT to Atezolizumab Plus Bevacizumab in Patients With Advanced Hepatocellular Carcinoma. Clin Nucl Med 2023;48:417-9. [Crossref] [PubMed]
- Kawamura Y, Kobayashi M, Shindoh J, et al. Pretreatment Positron Emission Tomography with 18F-Fluorodeoxyglucose May Be a Useful New Predictor of Early Progressive Disease following Atezolizumab plus Bevacizumab in Patients with Unresectable Hepatocellular Carcinoma. Oncology 2022;100:320-30. [Crossref] [PubMed]