
Lack of clinical benefit from preoperative short-term parenteral nutrition on the clinical prognosis of patients treated with radical gastrectomy for gastric cancer: a two-center retrospective study based on propensity score matching analysis
Highlight box
Key findings
• Preoperative short-term parenteral nutrition (PN) does not benefit clinical outcomes of patients treated with radical gastrectomy for gastric cancer (GC).
What is known and what is new?
• Preoperative long-term PN can improve the prognosis of abdominal surgery.
• Preoperative short-term PN support has no significant benefit on short-term postoperative complications or the long-term survival of patients with GC and may increase hospitalization costs.
What is the implication, and what should change now?
• Surgeons should carefully consider the necessity of preoperative PN and its duration in patients with an oral diet.
Introduction
Gastric cancer (GC), which ranks as the fifth most frequently diagnosed cancer and the fourth most frequent cause of cancer-related death worldwide (1), is a major contributor to the global burden of disability-adjusted life years, especially in East Asia, where both the incidence rates (32.1/100,000 for men; 13.2/100,000 for women) and mortality rates (15.9/100,000) are the highest (2,3). Surgery is the optimal choice for GC, but it is associated with high rates of postoperative complications and mortality (2). Effectively improving the prognosis of patients treated with surgery for GC has thus become an urgent and challenging issue. Many studies have identified factors that predict postoperative complications, including indicators based on body constitution, nutrition, inflammation, organ function, and hypercoagulation (4,5). Effectively improving the prognosis of patients treated with surgery for GC has thus become an urgent and challenging issue. A study has demonstrated that nutritional support can be used in conjunction with anticancer therapy to increase patients’ resistance to treatment (6). Patients with gastrointestinal (GI) tumors have an increased risk of malnutrition due to factors such as hypermetabolism, digestive and absorption dysfunction, and inflammatory response (7). Nutrition is crucial to improving the prognosis of patients after abdominal major surgery, particularly those with malnutrition and other issues affecting nutrition (8-15). It has been reported that nutritional support can improve body composition, muscle strength, weight, and reduce chemotherapy toxicity (16,17). Macronutrients such as fatty acids or amino acids and micronutrient components such as vitamin D also have anti-inflammatory properties (18).
Enteral nutrition (EN) and parenteral nutrition (PN) are supplements to oral diets that are administered to meet the nutritional needs of patients who lack adequate oral intake (19). The European Society for Parenteral and Enteral Nutrition (ESPEN) recommends EN for patients with cancer (20). In addition, as opposed to EN, PN is less commonly used by surgeons because it is less incompatible with human physiology (6). Studies have shown that the prevalence of postoperative complications decreases in malnourished patients with longer preoperative PN (≥7 days) (9), whereas it did not decrease with short-term PN (21,22). In fact, patients receiving PN therapy are not limited to those who are malnourished. The effect of short-term PN on the clinical prognosis of non-malnourished patients undergoing abdominal surgery is unknown, especially on long-term survival. Therefore, in this study, we investigated the short- and long-term prognosis of patients with GC undergoing radical surgery after receiving preoperative short-term PN support. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-1000/rc).
Methods
Patients
We retrospectively collected data of patients with GC who underwent surgery in the First Affiliated Hospital of Wenzhou Medical University and the Second Affiliated Hospital of Wenzhou Medical University in China from July 2014 to February 2019. In all cases, the following criteria were met: (I) age ≥18 years; (II) gastric adenocarcinoma confirmed by postoperative pathology; (III) undergoing radical gastrectomy; (IV) having abdominal computed tomography (CT) examination within 1 month prior to surgery; and (V) receiving no or only short-term PN (1–6 days) before surgery. We excluded patients with the following criteria: (I) lack of data required for analysis; (II) undergoing palliative or emergency surgery; (III) preoperative fasting due to illness; (IV) presence of severe immunologic, hematologic, or endocrine disease; and (V) GC concurrent with other malignancies. Finally, data from 1,155 patients, 557 of whom received short-term PN support before surgery, were included in the analysis.
Data collection
The following information was extracted from the medical record system or follow-up record for retrospective analysis: (I) basic clinical information, including age, gender, height, weight, Nutritional Risk Screening (NRS) 2002 score (23,24), Charlson comorbidity index (CCI) (25), preoperative hemoglobin level (with male hemoglobin <120 g/L or female <110 g/L considered to indicate anemia), and preoperative plasma albumin (<35 g/L was defined as hypoproteinemia); (II) tumor characteristics, including tumor histological classification, differentiation grade, and tumor-node-metastasis (TNM) staging [American Joint Committee on Cancer (AJCC) cancer staging system, eighth edition]; (III) surgical information, including surgical approach, extent of gastrectomy, method of digestive tract reconstruction mode, and combined organ resection; and (IV) prognosis, including postoperative complications, surgical mortality (death within 30 days after surgery), length of stay (LOS) following surgery, readmission rate within 1 month, long-term survival time, and total hospitalization costs. According to the Clavien-Dindo classification system, we included complications of grade II or higher in the analysis and defined severe complications as those of grade III or higher (26). After discharge, patients should have regular outpatient follow-up according to the doctor’s advice. If no outpatient records were found or the frequency was too low, telephone follow-up should be conducted. Within 2 years of discharge, patients were followed every 3 months and then every 6 months between 3 and 5 years after discharge until they lost contact or died.
Measurement of muscle mass
CT images of the lower edge of the third lumbar spine vertebra (L3) obtained from each patient within 1 month before surgery were saved. Two trained researchers used a specialized processing system (ImageJ; version 1.48V; Java 1.6.0-20; 64 bits; National Institutes of Health, Bethesda, MD, USA), all the skeletal muscles were delineated in Hounsfield units (HU) ranging from −29 to 150, and their areas were calculated. Subsequently, we divided muscle area by the square of height (m2) to obtain the L3 skeletal muscle index (SMI; cm2/m2). Based on our previous study, L3 SMI <34.9 cm2/m2 in females or SMI <40.8 cm2/m2 in males indicated a reduced muscle mass (low SMI) (27).
Preoperative nutrition support
Based on whether they received PN prior to surgery, the patients were divided into a non-PN group and a PN group. In the non-PN group, the normal hospital oral diet, including the special diet, was administered. The PN group received PN containing at least amino acids, glucose, and lipid emulsion for 1–6 days through a peripheral or central vein and could also include electrolytes, vitamins, and trace elements as a nutritional supplement to an oral diet (28).
Propensity score matching (PSM)
PSM is a statistical technique that employs intervention effect analysis in observational studies to lessen data bias and account for the confounding variables between groups. In order to increase the balance between groups, we used age, SMI, body mass index (BMI), NRS 2002 score, CCI, preoperative hemoglobin level, preoperative albumin level, tumor growth location, degree of differentiation, and TNM stage as matching factors, which may be variables that affect clinicians in making nutritional support decisions. PSM was implemented in a 1:1 proportion with the closest neighbor coordinating technique and a caliper of 0.02 being used to avoid mismatches.
Statistical analysis
All continuous data, including that of BMI, postoperative LOS, and hospital costs, were nonnormally distributed as determined by the Kolmogorov-Smirnov test. Consequently, the Mann-Whitney test was used to compare data between groups using the median and interquartile range (IQR). The Fisher exact or Pearson chi-squared tests were employed to compare categorical data, while the rank-sum test was applied for ordinal variables. Univariate analysis was used to examine the potential risk factors associated with complications or overall survival (OS). Variables with P<0.05 (two-tailed) were considered statistically significant, and results were subsequently presented as odds ratio (OR) or hazard ratio (HR) with 95% confidence intervals (CIs) in multivariate logistic or Cox regression analyses, respectively, using a “forward: LR” approach. Survival curves were compared using Kaplan-Meier analysis, and significance was determined using the log-rank test. P values <0.05 were considered statistically significant. SPSS software (version 25.0; IBM Corp., Armonk, NY, USA) was used to perform statistical analyses.
Ethical disclosure
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University (No. KY2022-202), and the Second Affiliated Hospital of Wenzhou Medical University was informed and agreed to this study. Individual consent for this retrospective analysis was waived.
Results
Demographic and therapeutic characteristics
A total of 1,155 patients were enrolled in this study, 557 (48.2%) of whom received short-term PN support before surgery. After PSM, a total of 478 patients were included in each group, and Table 1 lists the demographic and therapeutic characteristics before and after PSM. We found that patients in the PN group were older (age ≥70 years: 29.3% vs. 35.7%, P=0.019), had lower muscle mass (16.2% vs. 22.6%, P=0.006), higher nutritional risk (P<0.001), lower hemoglobin (26.3% vs. 38.1%, P<0.001), and albumin (15.7% vs. 23.5%, P=0.001). In addition, the PN group had deeper tumor invasion (T stage; P<0.001), more lymph node metastasis (N stage; P=0.047), and later phenomenon of TNM stage (P<0.001). There were no significant differences in gender, CCI, surgical approach, extent of resection, combined organ resection, or tumor location and differentiation between the two groups. The differences between the two groups were well homogenized after PSM.
Table 1
| Factors | All patients | After matching | |||||
|---|---|---|---|---|---|---|---|
| Non-PN group (n=598) | PN group (n=557) | P | Non-PN group (n=478) | PN group (n=478) | P | ||
| BMI (kg/m2) | 22.3 (20.4–24.3) | 22.3 (20.4–24.5) | 0.99 | 22.6 (20.6–24.6) | 22.4 (20.4–24.5) | 0.542 | |
| Age (years) | 0.019* | 0.451 | |||||
| <70 | 423 (70.7) | 358 (64.3) | 321 (67.2) | 323 (67.6) | |||
| ≥70 | 175 (29.3) | 199 (35.7) | 166 (34.7) | 155 (32.4) | |||
| Gender | 0.253 | 0.428 | |||||
| Female | 177 (29.6) | 148 (26.6) | 139 (29.1) | 128 (26.8) | |||
| Male | 421 (70.4) | 409 (73.4) | 339 (70.9) | 340 (71.1) | |||
| Low SMI | 0.006* | 0.741 | |||||
| No | 501 (83.8) | 431 (77.4) | 390 (81.6) | 386 (80.8) | |||
| Yes | 97 (16.2) | 126 (22.6) | 88 (18.4) | 92 (19.2) | |||
| NRS 2002 score | <0.001* | 0.470 | |||||
| 1–2 | 456 (76.3) | 357 (64.1) | 339 (70.9) | 327 (68.4) | |||
| 3–4 | 112 (18.7) | 162 (29.1) | 109 (22.8) | 124 (25.9) | |||
| 5–6 | 30 (5.0) | 38 (6.8) | 30 (6.3) | 27 (5.6) | |||
| CCI | 0.504 | 0.642 | |||||
| 0 | 360 (60.2) | 330 (59.2) | 281 (58.8) | 290 (60.7) | |||
| 1 | 163 (27.3) | 141 (25.3) | 127 (26.6) | 117 (24.5) | |||
| 2–6 | 75 (12.5) | 86 (15.4) | 70 (14.6) | 71 (14.9) | |||
| Preoperative anemia | <0.001* | 0.944 | |||||
| No | 441 (73.7) | 345 (61.9) | 331 (69.2) | 332 (69.5) | |||
| Yes | 157 (26.3) | 212 (38.1) | 147 (30.8) | 146 (30.5) | |||
| Preoperative hypoalbuminemia | 0.001* | 0.456 | |||||
| No | 504 (84.3) | 426 (76.5) | 384 (80.3) | 393 (82.2) | |||
| Yes | 94 (15.7) | 131 (23.5) | 94 (19.7) | 85 (17.8) | |||
| Scope of gastrectomy | 0.233 | 0.892 | |||||
| Partial | 399 (66.7) | 353 (63.4) | 308 (64.4) | 170 (35.6) | |||
| Total | 199 (33.3) | 204 (36.6) | 310 (64.9) | 168 (35.1) | |||
| Laparoscopy | 0.082 | 0.302 | |||||
| No | 390 (65.2) | 390 (70.0) | 313 (65.5) | 328 (68.6) | |||
| Yes | 208 (34.8) | 167 (30.0) | 165 (34.5) | 150 (31.4) | |||
| Combined organ excision | 0.894 | 0.561 | |||||
| No | 568 (95.0) | 530 (95.2) | 451 (94.4) | 455 (95.2) | |||
| Yes | 30 (5.0) | 27 (4.8) | 27 (5.6) | 23 (4.8) | |||
| Tumor location | 0.290 | 0.298 | |||||
| Pylorus | 373 (62.4) | 353 (63.4) | 293 (61.3) | 299 (62.6) | |||
| Body | 133 (22.2) | 129 (23.2) | 111 (23.2) | 113 (23.6) | |||
| Cardia | 85 (14.2) | 63 (11.3) | 68 (14.2) | 54 (11.3) | |||
| Linitis plastica | 7 (1.2) | 12 (2.2) | 6 (1.3) | 12 (2.5) | |||
| Degree of tumor differentiation | 0.435 | 0.540 | |||||
| Well-differentiated | 68 (11.4) | 52 (9.3) | 57 (11.9) | 51 (10.7) | |||
| Moderately differentiated | 129 (21.6) | 122 (21.9) | 104 (21.8) | 102 (21.3) | |||
| Poorly differentiated | 401 (67.1) | 383 (68.8) | 317 (66.3) | 325 (68.0) | |||
| T stage | <0.001* | 0.199 | |||||
| 1 | 230 (38.5) | 144 (25.9) | |||||
| 2 | 83 (13.9) | 89 (16.0) | 62 (13.0) | 83 (17.4) | |||
| 3 | 47 (7.9) | 31 (5.6) | 37 (7.7) | 27 (5.6) | |||
| 4 | 238 (39.8) | 293 (52.6) | 209 (43.7) | 224 (46.9) | |||
| N stage | 0.047* | 0.383 | |||||
| 0 | 323 (54.0) | 255 (45.8) | 254 (53.1) | 230 (48.1) | |||
| 1 | 77 (12.9) | 89 (16.0) | 62 (13.0) | 79 (16.5) | |||
| 2 | 82 (13.7) | 115 (20.6) | 71 (14.9) | 88 (18.4) | |||
| 3 | 116 (19.4) | 98 (17.6) | 91 (19.0) | 81 (16.9) | |||
| TNM stage | <0.001* | 0.325 | |||||
| I | 272 (45.5) | 184 (33.0) | 202 (42.3) | 182 (38.1) | |||
| II | 108 (18.1) | 132 (23.7) | 92 (19.2) | 105 (22.0) | |||
| III | 218 (36.5) | 241 (43.3) | 184 (38.5) | 191 (40.0) | |||
Values are presented as median (IQR) or n (%). *, P<0.05, indicating statistical significance. PN, parenteral nutrition; BMI, body mass index; SMI, skeletal muscle index; NRS 2002, Nutritional Risk Screening 2002; CCI, Charlson comorbidity index; TNM, tumor-node-metastasis; IQR, interquartile range.
Comparison of postoperative short-time outcomes between the PN group and non-PN group
As shown in Table 2, in the comparison of postoperative outcomes between the PN and non-PN groups, it was observed that hospitalization costs were higher in the PN group both before and after matching (pre-PSM: P<0.001; post-PSM: P<0.001), but there was no statistically significant in postoperative LOS (pre-PSM: P=0.092; post-PSM: P=0.460) or readmission rate within 30 days (pre-PSM: P=0.496; post-PSM P=0.793). In the analysis of postoperative complications, there was no statistical significance between the two groups in the incidence of total complications (pre-PSM: P=0.495; post-PSM: P>0.99) or infectious (pre-PSM: P=0.130; post-PSM: P=0.513) or noninfectious complications (pre-PSM: P=0.891; post-PSM: P=0.474). However, it was worth mentioning that anastomotic fistulas were more common in the PN group than in the non-PN group (pre-PSM: P=0.019; post-PSM, P=0.031).
Table 2
| Factors | All patients | After matching | |||||
|---|---|---|---|---|---|---|---|
| Non-PN group (n=598) | PN group (n=557) | P value | Non-PN group (n=478) | PN group (n=478) | P value | ||
| Postoperative LOS (days) | 13.0 (11.0–16.1) | 13.0 (11.0–18.1) | 0.092 | 13.0 (11.0–17.0) | 13.0 (11.0–18.1) | 0.460 | |
| Hospitalization cost† (¥) | 55,872.3 (48,897.2–65,283.1) |
60,564.6 (52,161.9–73,429.2) |
<0.001* | 55,953.1 (48,337.3–66,538.0) |
59,709.6 (51,452.9–72,971.4) |
<0.001* | |
| Readmission within 30 days | 39 (6.5) | 31 (5.6) | 0.496 | 32 (6.7) | 30 (6.3) | 0.793 | |
| Total complications‡ | 140 (23.4) | 140 (25.1) | 0.495 | 119 (24.9) | 119 (24.9) | >0.99 | |
| Clavien-Dindo grade | 0.427 | 0.886 | |||||
| Grade II | 90 (15.1) | 84 (15.1) | 79 (16.5) | 71 (14.9) | |||
| Grade III | 39 (6.5) | 40 (7.2) | 31 (6.5) | 38 (7.9) | |||
| Grade IV | 11 (1.8) | 12 (2.2) | 9 (1.9) | 9 (1.9) | |||
| Grade V | 0 (0.0) | 3 (0.5) | 0 (0.0) | 1 (0.2) | |||
| Infective complications | 70 (11.7) | 82 (14.7) | 0.130 | 63 (13.2) | 70 (14.6) | 0.513 | |
| Intra-abdominal infection | 28 (4.7) | 41 (7.4) | 0.055 | 27 (5.6) | 35 (7.3) | 0.293 | |
| Anastomotic leakage | 4 (0.7) | 13 (2.3) | 0.019* | 3 (0.6) | 11 (2.3) | 0.031* | |
| Duodenal stump leakage | 1 (0.2) | 4 (0.7) | 0.202 | 1 (0.2) | 4 (0.8) | 0.374 | |
| Incision infection | 12 (2.0) | 14 (2.5) | 0.562 | 11 (2.3) | 14 (2.9) | 0.543 | |
| Pancreatic fistula | 1 (0.2) | 4 (0.7) | 0.202 | 1 (0.2) | 4 (0.8) | 0.374 | |
| Pulmonary infections | 33 (5.5) | 27 (4.8) | 0.608 | 28 (5.9) | 22 (4.6) | 0.383 | |
| Sepsis | 0 (0.0) | 3 (0.5) | 0.112 | 0 (0.0) | 2 (0.4) | 0.499 | |
| Urinary infection | 0 (0.0) | 3 (0.5) | 0.112 | 0 (0.0) | 2 (0.4) | 0.499 | |
| Septic shock | 0 (0.0) | 3 (0.5) | 0.112 | 0 (0.0) | 3 (0.6) | 0.249 | |
| Noninfective complications | 93 (15.6) | 85 (15.3) | 0.891 | 78 (16.3) | 70 (14.6) | 0.474 | |
| Intra-abdominal bleeding | 21 (3.5) | 16 (2.9) | 0.538 | 17 (3.6) | 12 (2.5) | 0.346 | |
| Gastroparesis | 10 (1.7) | 12 (2.2) | 0.549 | 10 (2.1) | 9 (1.9) | 0.817 | |
| Intestinal obstruction | 18 (3.0) | 11 (2.0) | 0.261 | 17 (3.6) | 8 (1.7) | 0.068 | |
| Pleural effusion | 12 (2.0) | 12 (2.2) | 0.860 | 10 (2.1) | 9 (1.9) | 0.817 | |
| Peritoneal effusion | 4 (0.7) | 5 (0.9) | 0.746 | 1 (0.2) | 4 (0.8) | 0.374 | |
| Deep venous thrombosis | 8 (1.3) | 7 (1.3) | 0.903 | 6 (1.3) | 7 (1.5) | 0.780 | |
| Respiratory failure | 2 (0.3) | 3 (0.5) | 0.677 | 2 (0.4) | 3 (0.6) | >0.99 | |
| Cardiac insufficiency | 2 (0.3) | 3 (0.5) | 0.677 | 2 (0.4) | 3 (0.6) | >0.99 | |
| Hypohepatia | 3 (0.5) | 8 (1.4) | 0.102 | 3 (0.6) | 7 (1.5) | 0.204 | |
| Renal insufficiency | 1 (0.2) | 1 (0.2) | >0.99 | 1 (0.2) | 1 (0.2) | >0.99 | |
| Multiple organ dysfunction syndrome | 0 (0.0) | 2 (0.4) | 0.232 | 0 (0.0) | 2 (0.4) | 0.499 | |
| Lymphatic fistula | 6 (1.0) | 6 (1.1) | 0.902 | 4 (0.8) | 5 (1.0) | >0.99 | |
| Others | 7 (1.2) | 6 (1.1) | 0.881 | 5 (1.0) | 4 (0.8) | >0.99 | |
| Death | 0 (0.0) | 3 (0.5) | 0.112 | 0 (0.0) | 1 (0.2) | >0.99 | |
Values are presented as median (IQR) or n (%). †, total complications were defined as complications classified as grade II or above; *, P<0.05, indicating statistical significance. PN, parenteral nutrition; LOS, length of stay; IQR, interquartile range.
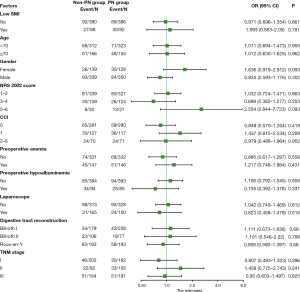
Subgroup analysis was then conducted on the influence of preoperative short-term PN support in order to identify potential beneficiaries, but unfortunately, our results (Figure 1) showed that short-term PN support did not demonstrate meaningful effectiveness in the subgroups, including in those of age, gender, NRS 2002 score, CCI, anemia, hypoalbuminemia, surgical method, and tumor TNM stage.
Comparison of OS between the PN group and non-PN group
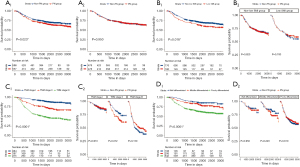
We further explored whether preoperative short-term PN had an impact on OS (Figure 2). As shown in Figure 2A, before the PSM, the PN group had a lower OS rate than did the non-PN group (P=0.023), but this difference disappeared after matching (P=0.950). In addition, we found that the survival of low-SMI patients was lower than that of normal SMI patients (Figure 2B1, P=0.018), but in the subgroup analysis, preoperative short-term PN had no obvious effect on the survival of any of the groups (Figure 2B2; non-low SMI group: P=0.820; low SMI group: P=0.700). Similarly, survival rates differed significantly across patients with different TNM stages (P<0.0001) or different degrees of tumor differentiation (P<0.0001), but these differences were not apparent in the subgroup analysis (Figure 2C,2D).
Risk factors for postoperative complications and OS
Multivariate logistic analysis was used to determine factors associated with overall postoperative complications (Table 3). Univariate analysis showed that low SMI (P=0.020), age ≥70 years (P=0.003), higher CCI (P=0.007), preoperative anemia (P<0.001), hypoalbuminemia (P=0.006), laparoscopic surgery (P<0.001) and the digestive tract reconstruction method (P=0.001) were substantially correlated with total postoperative complications. After these factors were incorporated into the subsequent multivariate analysis, age ≥70 years (P=0.043), higher CCI (P=0.012), preoperative anemia (P=0.004), laparoscopic surgery (P=0.002), and digestive tract reconstruction mode (P=0.005) were found to be independently associated with the incidence of postoperative complications in this study.
Table 3
| Factors | Without complications (n=718) | With complications (n=238) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Group | >0.99 | ||||||
| Non-PN group | 359 (50.0) | 119 (50.0) | 1 | ||||
| PN group | 359 (50.0) | 119 (50.0) | 1.000 (0.746–1.341) | ||||
| Low SMI | 0.020* | 0.114 | |||||
| No | 595 (82.9) | 181 (76.1) | 1 | ||||
| Yes | 123 (17.1) | 57 (23.9) | 1.523 (1.068–2.173) | ||||
| Age (years) | 0.003* | 0.043* | |||||
| <70 | 496 (69.1) | 139 (58.4) | 1 | 1 | |||
| ≥70 | 222 (30.9) | 99 (41.6) | 1.591 (1.176–2.153) | 1.380 (1.010–1.886) | |||
| Gender | 0.362 | ||||||
| Female | 206 (28.7) | 61 (25.6) | 1 | ||||
| Male | 512 (71.3) | 177 (74.4) | 1.167 (0.837–1.629) | ||||
| NRS 2002 score | 0.105 | ||||||
| 1–2 | 505 (70.3) | 161 (67.6) | 1 | ||||
| 3–4 | 177 (24.7) | 56 (23.5) | 0.992 (0.700–1.407) | ||||
| 5–6 | 36 (5.0) | 21 (8.8) | 1.830 (1.038–3.225) | ||||
| CCI | 0.007* | 0.012* | |||||
| 0 | 447 (62.3) | 124 (52.1) | 1 | 1 | |||
| 1 | 178 (24.8) | 66 (27.7) | 1.337 (0.946–1.888) | 1.345 (0.943–1.918) | |||
| 2–6 | 93 (13.0) | 48 (20.2) | 1.861 (1.246–2.779) | 1.815 (1.202–2.741) | |||
| Preoperative hypoalbuminemia | 0.006* | 0.375 | |||||
| No | 598 (83.3) | 179 (75.2) | 1 | ||||
| Yes | 120 (16.7) | 59 (24.8) | 1.643 (1.153–2.339) | ||||
| Preoperative anemia | <0.001* | 0.004* | |||||
| No | 521 (72.6) | 142 (59.7) | 1 | 1 | |||
| Yes | 197 (27.4) | 96 (40.3) | 1.788 (1.316–2.429) | 1.605 (1.167–2.206) | |||
| Laparoscope | <0.001* | 0.002* | |||||
| No | 458 (63.8) | 183 (76.9) | 1 | 1 | |||
| Yes | 260 (36.2) | 55 (23.1) | 0.529 (0.378–0.742) | 0.580 (0.410–0.822) | |||
| Digestive tract reconstruction mode | 0.001* | 0.005* | |||||
| Billroth I | 310 (43.2) | 77 (32.4) | 1 | 1 | |||
| Billroth II | 142 (19.8) | 41 (17.2) | 1.162 (0.758–1.783) | 0.969 (0.624–1.506) | |||
| Roux-en-Y | 266 (37.0) | 120 (50.4) | 1.816 (1.306–2.526) | 1.639 (1.169–2.298) | |||
| Combined organ excision | 0.126 | ||||||
| No | 685 (95.4) | 221 (92.9) | 1 | ||||
| Yes | 33 (4.6) | 17 (7.1) | 1.597 (0.872–2.922) | ||||
| Degrees of tumor differentiation | 0.904 | ||||||
| Well-differentiated | 83 (11.6) | 25 (10.5) | 1 | ||||
| Moderately differentiated | 154 (21.4) | 52 (21.8) | 1.121 (0.649–1.936) | ||||
| Poorly differentiated | 481 (67.0) | 161 (67.6) | 1.111 (0.687–1.798) | ||||
| TNM stage | 0.083 | ||||||
| I | 303 (42.2) | 81 (34.0) | 1 | ||||
| II | 142 (19.8) | 55 (23.1) | 1.449 (0.975–2.153) | ||||
| III | 273 (38.0) | 102 (42.9) | 1.398 (1.000–1.953) | ||||
If not specifically stated, the values are presented as n (%). *, P<0.05, indicating statistical significance. OR, odds ratio; CI, confidence interval; PN, parenteral nutrition; SMI, skeletal muscle index; NRS 2002, Nutritional Risk Screening 2002; CCI, Charlson comorbidity index; TNM, tumor-node-metastasis.
Cox analysis was used to determine the risk factors affecting postoperative OS (Table 4). Significant factors om univariate analysis including low SMI (P=0.019), age (P<0.001), NRS 2002 score (P=0.002), preoperative anemia (P<0.001), hypoalbuminemia (P<0.001), laparoscopic surgery (P<0.001), digestive tract reconstruction mode (P<0.001), combined organ excision (P<0.001), degrees of tumor differentiation (P<0.001), and TNM stage (P<0.001) were included in multifactor analysis, after which only age ≥70 years (P<0.001), laparoscopic surgery (P=0.008), digestive tract reconstruction mode (P<0.001), combined organ excision (P=0.031), and degree of differentiation (P=0.002), and TNM stage (P<0.001) were found to affect OS.
Table 4
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Group | 0.949 | ||||
| Non-PN group | 1 | ||||
| PN group | 1.007 (0.805–1.261) | ||||
| Low SMI | 0.019* | 0.450 | |||
| No | 1 | ||||
| Yes | 1.374 (1.055–1.79) | ||||
| Age (years) | <0.001* | <0.001* | |||
| <70 | 1 | 1 | |||
| ≥70 | 1.682 (1.341–2.11) | 1.604 (1.274–2.02) | |||
| Gender | 0.084 | ||||
| Female | 1 | ||||
| Male | 1.261 (0.969–1.64) | ||||
| NRS 2002 score | 0.002* | 0.571 | |||
| 1–2 | 1 | ||||
| 3–4 | 1.318 (1.02–1.705) | ||||
| 5–6 | 1.933 (1.292–2.892) | ||||
| CCI | 0.558 | ||||
| 0 | 1 | ||||
| 1 | 1.039 (0.796–1.356) | ||||
| 2–6 | 1.189 (0.869–1.628) | ||||
| Preoperative hypoalbuminemia | <0.001* | 0.247 | |||
| No | 1 | ||||
| Yes | 1.858 (1.445–2.389) | ||||
| Preoperative anemia | <0.001* | 0.073 | |||
| No | 1 | ||||
| Yes | 1.942 (1.548–2.437) | ||||
| Laparoscopy | <0.001* | 0.008* | |||
| No | 1 | 1 | |||
| Yes | 0.498 (0.378–0.656) | 0.684 (0.515–0.907) | |||
| Digestive tract reconstruction | <0.001* | <0.001* | |||
| Billroth I | 1 | 1 | |||
| Billroth II | 1.888 (1.354–2.633) | 1.256 (0.893–1.764) | |||
| Roux-en-Y | 2.575 (1.971–3.364) | 1.727 (1.312–2.273) | |||
| Combined organ excision | <0.001* | 0.031* | |||
| No | 1 | 1 | |||
| Yes | 2.302 (1.57–3.375) | 1.536 (1.041–2.267) | |||
| Degrees of tumor differentiation | <0.001* | 0.002* | |||
| Well differentiated | 1 | 1 | |||
| Moderately differentiated | 1.581 (0.893–2.797) | 0.800 (0.44–1.453) | |||
| Poorly differentiated | 3.162 (1.906–5.245) | 1.394 (0.807–2.408) | |||
| TNM stage | <0.001* | <0.001* | |||
| I | 1 | 1 | |||
| II | 2.658 (1.807–3.908) | 2.146 (1.433–3.213) | |||
| III | 5.996 (4.373–8.222) | 4.275 (3.016–6.058) | |||
*, P<0.05, indicating statistical significance. OS, overall survival; HR, hazard ratio; CI confidence interval; PN, parenteral nutrition; SMI, skeletal muscle index; NRS 2002, Nutritional Risk Screening 2002; CCI, Charlson comorbidity index; TNM, tumor-node-metastasis.
Discussion
Patients with GC may have abnormalities such as elevated basal metabolic rate, carbohydrate metabolism, and protein metabolism (7). Surgery can cause a series of stress reactions, such as the release of cytokines, including stress hormones and inflammation mediators, that enhance glycogen, fat, and protein catabolism and release sufficient substrates to facilitate healing (29). These reactions lead to insulin resistance, immunosuppression, muscle mass loss, and other adverse effects (30). Proper nutrition can provide adequate raw materials and mobilize the required substrates for anabolism during rapid convalescence (31). The main goal of perioperative nutritional therapy is to enable patients to obtain adequate energy reserves in the shortest time; to better maintain muscle mass, immunity, and cognitive function by avoiding starvation; and to facilitate postoperative recovery from surgical trauma and possible infections. Compared with PN, EN is favored by many nutritionists and surgeons because the way of absorption and utilization of nutrients is more in line with human physiology and helps to maintain the integrity of the intestinal mucosal structure and barrier function (6). Although oral intake is the preferred form of nutritional intake, PN is needed to provide nutritional support for patients with upper GI cancer who experience nausea, vomiting, dysphagia, obstruction, and other phenomena hindering eating. Therefore, preoperative PN nutrition is still extensively used.
A randomized open trial conducted by Sánchez-Guillén et al. (32) indicated that perioperative PN appeared to be statistically associated with fewer complications in patients with colorectal cancer (OR =0.2; 95% CI: 0.08–0.87), and a randomized clinical trial subanalysis by López-Rodríguez-Arias et al. (33) reported a 15% reduction in complications in patients with low SMI supported by peripheral PN, but these were for perioperative PN (1 day prior to surgery to 3 days after surgery). A recent review focusing on perioperative nutrition in GI surgery demonstrated that there were insufficient studies on preoperative PN, especially randomized controlled clinical trials (32), and further studies will be beneficial to guiding clinical practice. Some studies have found that receiving long-term PN (≥7 days) before surgery reduces postoperative complications, especially malnutrition (11-14,34). In a prospective study, preoperative and total length of hospital stay were longer in the preoperative nutrition group (median LOS: 33 vs. 27 days, P<0.001) (35). Considering the actual situation of hospitals in China, only a few patients with GC clinically receive long-term preoperative nutritional support. Because few studies have investigated the effect of short-term intravenous nutrition interventions on clinical outcomes, the importance of preoperative short-term nutrition is unclear.
The effect of PN on patients with malnutrition has been demonstrated, however the effect on well-nourished patients has not been studied. Our data showed that there were also patients with better nutrition who received short-term PN. The incidence of true malnutrition in preoperative patients may be much higher than described before surgery (36), especially in weak and elderly patients. These patients often only have decreased appetite, insufficient food absorption, conversion and utilization, fatigue, mild abnormal blood indicators, which are high risks of malnutrition without showing significant weight loss, so the doctor in charge may offer them nutritional support. Our study found that patients in the PN group were older and had a lower muscle mass, lower hemoglobin and albumin levels, and a later tumor stage, which made patients more susceptible to malnutrition; moreover, the PN group had shorter survival time (P=0.023). In the analysis of risk factors for postoperative complications, we found that advanced age (≥70 years old), CCI 2–6 points, and open surgery and Roux-en-Y anastomosis of the digestive tract increase the probability of postoperative complications, which may be due to the increase of operation complexity, prolonged operation time, greater trauma to the human body, and stronger stress response. However, the factors mentioned above often cannot be changed, increasing the difficulty of clinical intervention. Cox regression analysis of survival showed that age ≥70 years, Roux-en-Y anastomosis, combined organ excision, low differentiation, and high TNM stage were independent risk factors for reducing postoperative survival, which was similar to our previous finding (37). PN did not affect long-term survival (P=0.950) regardless of whether patients were grouped according to SMI, TNM stage, or tumor differentiation. Huang et al. and Xu et al. demonstrated that short-term preoperative PN (3–7 days) did not significantly improve the prognosis of patients with GC at high risk of malnutrition or sarcopenia (21,22), which is similar to our results. We also found no significant effect of short-term preoperative PN on long-term survival (P=0.950). One possible reason for this is that the physiological functions of the human body do not recover within the first 7 days after PN. Due to the short duration of preoperative nutritional support, nutritional reserves may be insufficient for supporting protein synthesis, immune response, and acute wound healing (11). The patients most likely to benefit from preoperative PN are those least likely to tolerate oral administration and/or EN. However, we excluded patients who had to fast due to bleeding, perforation, or acute obstruction and those who were in urgent need of PN, thereby reducing the effect of PN.
Malnutrition is closely related to sarcopenia, and they share many pathophysiological components (38-40). Sarcopenia has been recognized as an independent risk factor for postoperative complications and may increase the risk of total and major complications after GI tumor resection by approximately 30% to 40% (27,41-43). This study did not find muscle mass or high risk of malnutrition to be independent risk factors for postoperative complications. Since this study was conducted in China, most patients with GC underwent preoperative CT scans, and CT is considered one of the gold standards for measuring muscle mass (44). The 19.3% prevalence of low muscle mass and 29.6% prevalence of high risk of malnutrition in our study are somewhat lower than those in our previous reports (22,27). This may be due to the fact that we excluded patients who received PN for at least 7 days before surgery, and these patients might have a higher probability of reduced muscle mass and a higher risk of malnutrition. In addition, muscle function indicators such as strength and gait speed, which may reduce the impact of sarcopenia on prognosis, were not included in this study. Subgroup analysis showed that PN support did not effectively improve the short-term prognosis of various patients after surgery. However, it may be beneficial in patients with albumin levels <35 g/L, open surgery, Roux-en-Y anastomosis, or TNM stage III, but there was no statistically significant difference. Amino acid preparations are important components of intravenous nutritional supplement that promote protein synthesis in the body. A higher serum albumin level may be a protective factor for improved surgical complications and survival (45). Further analysis is needed to determine whether long-term PN is beneficial to the prognosis of these patients.
Many factors known to influence clinical outcomes, such as age, diabetes, and other chronic diseases, cannot or are difficult to change preoperatively, and nutritional support is easily modifiable by the surgeon. For patients undergoing GI cancer surgery, improved physiological reserve means greater adaptability and resilience to surgical stress. However, PN seems to be used inappropriately in many elective abdominal procedures. We found that in patients who could tolerate oral feeding, clinical results were not significantly improved by preoperative short-term PN, and in the absence of clinical benefit, PN increased the economic burden of patients (post-PSM: ¥55,953.1 vs. ¥59,709.6, P<0.001), which is contrary to the principle of efficient health care resource utilization. Therefore, it is important for clinicians to solidly grasp the indications of preoperative nutritional therapy, and short-term PN support should not be the first choice.
Our study also had some limitations. First, only two centers and a limited number of patients were employed in the study. Second, PSM only controlled for the effects of measurable variables and to some extent the effects of confounding factors, but there were still some unmeasured confounders that could have introduced bias. In addition, PSM reduced the sample size, which changed the characteristics of the population to a certain extent and decreased the representativeness of the sample. Therefore, multicenter, prospective, randomized trials are urgently needed to confirm our conclusions.
Conclusions
Short-term preoperative PN does not improve postoperative clinical outcomes in patients with GC and may even increase the economic burden. PN is not recommended for patients who can tolerate oral feeding, and a firmer understanding of the indications and duration of preoperative nutritional support is needed.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-1000/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-1000/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-1000/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-1000/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University (No. KY2022-202) and the Second Affiliated Hospital of Wenzhou Medical University was informed and agreed to this study. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Kim Y, Ejaz A, Spolverato G, et al. Conditional survival after surgical resection of gastric cancer: a multi-institutional analysis of the us gastric cancer collaborative. Ann Surg Oncol 2015;22:557-64. [Crossref] [PubMed]
- Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol 2020;18:534-42. [Crossref] [PubMed]
- Kanda M. Preoperative predictors of postoperative complications after gastric cancer resection. Surg Today 2020;50:3-11. [Crossref] [PubMed]
- Copeland EM 3rd. Historical perspective on nutritional support of cancer patients. CA Cancer J Clin 1998;48:67-8. [Crossref] [PubMed]
- Xi X, Yang MX, Wang XY, et al. Predictive value of prognostic nutritional index on infection after radical gastrectomy: a retrospective study. J Gastrointest Oncol 2022;13:569-80. [Crossref] [PubMed]
- Dempsey DT, Feurer ID, Knox LS, et al. Energy expenditure in malnourished gastrointestinal cancer patients. Cancer 1984;53:1265-73. [Crossref] [PubMed]
- Bellantone R, Doglietto GB, Bossola M, et al. Preoperative parenteral nutrition in the high risk surgical patient. JPEN J Parenter Enteral Nutr 1988;12:195-7. [Crossref] [PubMed]
- Jie B, Jiang ZM, Nolan MT, et al. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition 2012;28:1022-7. [Crossref] [PubMed]
- Burden S, Todd C, Hill J, et al. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev 2012;11:CD008879. [Crossref] [PubMed]
- Lakananurak N, Gramlich L. The Role of Preoperative Parenteral Nutrition. Nutrients 2020;12:1320. [Crossref] [PubMed]
- Müller JM, Brenner U, Dienst C, et al. Preoperative parenteral feeding in patients with gastrointestinal carcinoma. Lancet 1982;1:68-71. [Crossref] [PubMed]
- Durán-Poveda M, Suárez-de-la-Rica A, Cancer Minchot E, et al. Knowledge and Practices of Digestive Surgeons concerning Specialized Nutritional Support in Cancer Patients: A Survey Study. Nutrients 2022;14:4764. [Crossref] [PubMed]
- Kamocki Z, Matowicka-Karna J, Jurczuk A, et al. Preoperative Glutamine Supplementation in Gastric Cancer-Thrombocyte Phagocytic Activity and Early Postoperative Outcomes. Nutrients 2023;15:2911. [Crossref] [PubMed]
- Thibault R, Abbasoglu O, Ioannou E, et al. ESPEN guideline on hospital nutrition. Clin Nutr 2021;40:5684-709. [Crossref] [PubMed]
- Cereda E, Turri A, Klersy C, et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med 2019;8:6923-32. [Crossref] [PubMed]
- Caccialanza R, Cereda E, Caraccia M, et al. Early 7-day supplemental parenteral nutrition improves body composition and muscle strength in hypophagic cancer patients at nutritional risk. Support Care Cancer 2019;27:2497-506. [Crossref] [PubMed]
- Stumpf F, Keller B, Gressies C, et al. Inflammation and Nutrition: Friend or Foe? Nutrients 2023;15:1159. [Crossref] [PubMed]
- Worthington P, Balint J, Bechtold M, et al. When Is Parenteral Nutrition Appropriate? JPEN J Parenter Enteral Nutr 2017;41:324-77. [Crossref] [PubMed]
- Weimann A, Braga M, Carli F, et al. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr 2017;36:623-50. [Crossref] [PubMed]
- Huang ZX, Zhang HH, Zhang WT, et al. Effect of Short-Term Preoperative Parenteral Nutrition Support for Gastric Cancer Patients with Sarcopenia: a Propensity Score Matching Analysis. J Gastrointest Surg 2022;26:1362-72. [Crossref] [PubMed]
- Xu LB, Huang ZX, Zhang HH, et al. Impact of Preoperative Short-Term Parenteral Nutrition Support on the Clinical Outcome of Gastric Cancer Patients: A Propensity Score Matching Analysis. JPEN J Parenter Enteral Nutr 2021;45:729-37. [Crossref] [PubMed]
- Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321-36. [Crossref] [PubMed]
- Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415-21. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Zhuang CL, Huang DD, Pang WY, et al. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine (Baltimore) 2016;95:e3164. [Crossref] [PubMed]
- American Gastroenterological Association medical position statement: parenteral nutrition. Gastroenterology 2001;121:966-9.
- Shinsyu A, Bamba S, Kurihara M, et al. Inflammatory cytokines, appetite-regulating hormones, and energy metabolism in patients with gastrointestinal cancer. Oncol Lett 2020;20:1469-79. [Crossref] [PubMed]
- Manou-Stathopoulou V, Korbonits M, Ackland GL. Redefining the perioperative stress response: a narrative review. Br J Anaesth 2019;123:570-83. [Crossref] [PubMed]
- Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466-77. [Crossref] [PubMed]
- Sánchez-Guillén L, Soriano-Irigaray L, López-Rodríguez-Arias F, et al. Effect of Early Peripheral Parenteral Nutrition Support in an Enhanced Recovery Program for Colorectal Cancer Surgery: A Randomized Open Trial. J Clin Med 2021;10:3647. [Crossref] [PubMed]
- López-Rodríguez-Arias F, Sánchez-Guillén L, Lillo-García C, et al. Assessment of Body Composition as an Indicator of Early Peripheral Parenteral Nutrition Therapy in Patients Undergoing Colorectal Cancer Surgery in an Enhanced Recovery Program. Nutrients 2021;13:3245. [Crossref] [PubMed]
- Heyland DK, Montalvo M, MacDonald S, et al. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg 2001;44:102-11.
- Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr 2000;24:7-14. [Crossref] [PubMed]
- Dolan RD, Almasaudi AS, Dieu LB, et al. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle 2019;10:111-22. [Crossref] [PubMed]
- Xu LB, Zhang HH, Shi MM, et al. Metabolic syndrome-related sarcopenia is associated with worse prognosis in patients with gastric cancer: A prospective study. Eur J Surg Oncol 2020;46:2262-9. [Crossref] [PubMed]
- Schneider SM, Correia MITD. Epidemiology of weight loss, malnutrition and sarcopenia: A transatlantic view. Nutrition 2020;69:110581. [Crossref] [PubMed]
- Sieber CC. Malnutrition and sarcopenia. Aging Clin Exp Res 2019;31:793-8. [Crossref] [PubMed]
- Kiss N, Bauer J, Boltong A, et al. Awareness, perceptions and practices regarding cancer-related malnutrition and sarcopenia: a survey of cancer clinicians. Support Care Cancer 2020;28:5263-70. [Crossref] [PubMed]
- Zhou CJ, Zhang FM, Zhang FY, et al. Sarcopenia: a new predictor of postoperative complications for elderly gastric cancer patients who underwent radical gastrectomy. J Surg Res 2017;211:137-46. [Crossref] [PubMed]
- Fukuda Y, Yamamoto K, Hirao M, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer 2016;19:986-93. [Crossref] [PubMed]
- Simonsen C, de Heer P, Bjerre ED, et al. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann Surg 2018;268:58-69. [Crossref] [PubMed]
- Tagliafico AS, Bignotti B, Torri L, et al. Sarcopenia: how to measure, when and why. Radiol Med 2022;127:228-37. [Crossref] [PubMed]
- Xu JY, Tian XD, Song JH, et al. Preoperative Nutrition Support May Reduce the Prevalence of Postoperative Pancreatic Fistula after Open Pancreaticoduodenectomy in Patients with High Nutritional Risk Determined by NRS2002. Biomed Res Int 2021;2021:6691966. [Crossref] [PubMed]


