
The influencing factors for tumor thrombus in patients with hepatocellular carcinoma
Highlight box
Key findings
• Ki-67 expression level was one of the independent predictors of tumor thrombus in liver cancer.
What is known and what is new?
• Age, cirrhosis, alpha fetoprotein (AFP) level, tumor number, boundary and diameter of tumor, and peritumoral enhancement were associated with the occurrence of microvascular invasion (MVI) in liver cancer.
• The combination of tumor diameter and Ki-67 expression level demonstrated high diagnostic efficacy for tumor thrombus in liver cancer.
What is the implication, and what should change now?
• We believe these factors (Ki-67 expression level, AFP level, tumor number, boundary and diameter of tumor, and peritumoral enhancement) can serve as a key index for predicting the formation of tumor thrombus and the prognosis of patients with hepatocellular carcinoma (HCC), thus better informing clinical decision-making.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and has the second highest level of mortality rate among all cancers (1). Although surgery is considered to be the most effective treatment, HCC in the majority of patients is accompanied by intrahepatic metastasis, tumor thrombus, and ascites, leading to these patients being unable to undergo surgery (2). Tumor thrombus is one of key factors in determining suitability for surgery and includes microvascular invasion (MVI) and portal vein tumor thrombus (PVTT). Mähringer-Kunz et al. (3). reported that patients with HCC and tumor thrombus experience a very poor prognosis even when the tumor thrombus is in the early stage. Therefore, greater attention should be paid to the evolution and development of tumor thrombus. PVTT can be diagnosed by imaging methods, while the diagnosis of MVI is dependent upon pathology. MVI is considered to be an important factor in the early invasion and metastasis of liver cancer (4,5), with the incidence in liver cancer ranging from 15% to 57.1% (6-9), and the survival rate of HCC patients with MVI is much lower than that of patients without MVI. Therefore, it is crucial to accurately predict the independent predictors of tumor thrombus formation. The purpose of this study was thus to identify the influencing factors of tumor thrombus formation in patients with HCC. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-983/rc).
Methods
Patients
From August 2011 to December 2022, 240 consecutive patients diagnosed with HCC in The First Hospital of Jiaxing were retrospectively enrolled in the study. The diagnostic criteria of HCC were based on the American Association for the Study of Liver Diseases (AASLD). Among them, nine patients were excluded due to preoperative treatment or incomplete imaging data, and a total of 231 patients were eventually enrolled in this study (Figure 1). This study was approved by the Ethics Committee of The First Hospital of Jiaxing (approval No. LS2021-KY-383). Informed consent of patients was not required for this study due to the retrospective nature of the design. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
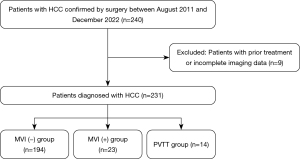
The inclusion criteria were as follows: (I) patients who underwent surgical treatment or needle biopsy in our hospital; (II) patients who were diagnosed with HCC as confirmed by pathology and immunohistochemistry; and (III) patients who underwent computed tomography (CT) or enhanced magnetic resonance (MR) imaging examination, with the images being usable for analysis. Meanwhile, the exclusion criteria were as follows: (I) incomplete clinical and examination data; (II) imaging examinations not performed in our hospital; and (III) patients who received transcatheter arterial chemoembolization (TACE) or radiofrequency ablation (RFA) before surgical treatment.
Definition
The diagnostic criterion of MVI was cancer cell nests with surrounding endothelial cells found in microvascular pathology (10).
The diagnostic criteria of PVTT included solid space-occupying lesions in the portal vein found in all phases on enhanced CT or MR images and partial enhancement and filling defects observed in the arterial phase and portal phase (Figure 2A-2C), respectively.
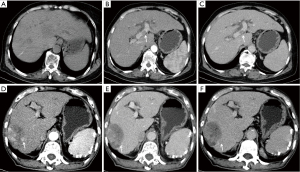
Peritumoral enhancement was defined as the focal patch of the lesion enhanced in the arterial phase, showing isointensity or isodensity in the portal vein phase and the delayed phase (Figure 2D-2F).
The Ki-67 index is determined by the proportion of Ki-67 positive cells in the total number of cells, when the nuclei and/or cytoplasm of Ki-67 positive cells are tan or brownish yellow. Ki-67 specific classification is: <10% as negative (−), 10% to 19% as weak positive (+), 20% to 49% as positive (++), and more than 50% as strong positive (+++).
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software (IBM Corp). MVI-negative status, MVI-positive status, and PVTT were considered to be the dependent variables; gender, age, tumor diameter, tumor quantity, Ki-67, AFP and alanine aminotransferase levels, among others, were considered to be the independent variables. The data of patients with HCC were analyzed with univariate analysis. (I) The measurement data were analyzed with the Kolmogorov-Smirnov (K-S) test to determine whether they conformed to a normal distribution. If the data followed a normal distribution, a t-test or F test was used to determine whether there were differences between the three independent samples, with these data being expressed as the mean ± standard deviation. If the data did not follow a normal distribution, the nonparametric rank-sum test was used, with these data being expressed as the median and range. (II) The categorical data were tested with the χ2 or Fisher exact test and are expressed as frequency or percentage. (III) Factors with P<0.05 in the univariate analysis were included in the multiple logistic regression model to further analyze the independent risk factors affecting the formation of tumor thrombus. (IV) A receiver operating characteristic (ROC) curve was drawn to predict the threshold of tumor thrombus formation. P<0.05 was considered statistically significant.
Results
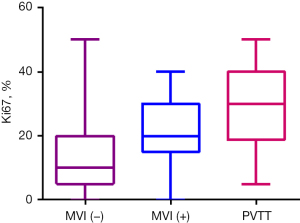
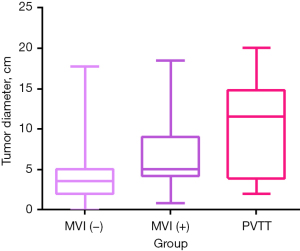
According to the inclusion criteria, 178 males and 53 females with a mean age of 60 years (range, 26–88 years) were included in this study; the numbers of patients in the MVI-negative, MVI-positive, and PVTT groups were 194, 23, and 14, respectively (Table 1). Ki-67 expression level in the MVI-positive and PVTT groups was higher than that in the MVI-negative group (Figure 3), while tumor diameter in the PVTT group was higher than that in the MVI-negative and MVI-positive groups (Figure 4).
Table 1
| Characteristics | Values |
|---|---|
| Gender | |
| Male | 178 (77.1) |
| Female | 53 (22.9) |
| Age (years), median [range] | 60 [26–88] |
| MVI (–) | 194 (84.0) |
| MVI (+) | 23 (10.0) |
| PVTT | 14 (6.1) |
| Liver cirrhosis | 101 (43.7) |
| Hepatitis B virus | 89 (38.5) |
| Other | 142 (61.5) |
| Ki-67 (%) | 16.8±12.8 |
| AFP (ng/mL) | 289.3±455.3 |
| Extrahepatic metastasis | 28 |
| Tumor size (cm) | 4.6±3.7 |
Data are expressed as mean ± standard deviation, median [range], number or number (percentage). HCC, hepatocellular carcinoma; MVI, microvascular invasion; PVTT, portal vein tumor thrombosis; AFP, alpha fetoprotein.
Univariate analysis of tumor thrombus grades in patients with HCC
Univariate analysis revealed statistically significant differences in eight characteristics [tumor diameter, alpha fetoprotein (AFP) level, Ki-67 expression level, gender, tumor quantity, arteriovenous fistula, peritumoral enhancement, and satellite nodules] among the three groups (P<0.05) (Tables 2,3).
Table 2
| Parameter | MVI (−) (n=194) | MVI (+) (n=23) | PVTT (n=14) | P |
|---|---|---|---|---|
| Diameter (cm) | 3.5 [2–5] | 5 [4.2–9] | 11.5 [3.9–14.7] | <0.001 |
| AFP (ng/mL) | 19.2 [4.3–194.5] | 118.5 [24.6–1,210] | 47.05 [4.8–1,210] | 0.006 |
| Ki-67 (%) | 10 [5–20] | 20 [15–30] | 30 [18–40] | <0.001 |
| Age (years) | 62±11 | 58.6±12.9 | 55.9±12.3 | 0.098 |
| ALT (U/L) | 35 [24–71.5] | 41 [24–68] | 60 [4.1–82.5] | 0.235 |
| CEA (ng/mL) | 3 [1.8–4.4] | 2.7 [1.3–4.5] | 2.15 [1.5–4.1] | 0.309 |
| CA19-9 (U/mL) | 17.55 [10.3–34.9] | 16.9 [6.7–32.6] | 21.05 [14.9–145.4] | 0.143 |
Data are expressed as mean ± standard deviation or median [range]. HCC, hepatocellular carcinoma; MVI, microvascular invasion; PVTT, portal vein tumor thrombosis; AFP, alpha fetoprotein; ALT, alanine transaminase; CEA, carcinoembryonic antigen; CA19-9, cancer antigen 19-9.
Table 3
| Parameters | Sum (n=231) | MVI (−) (n=194) | MVI (+) (n=23) | PVTT (n=14) | χ2 | P |
|---|---|---|---|---|---|---|
| Gender, n (%) | 5.971 | 0.038 | ||||
| 0, male | 178 (77.1) | 144 (74.2) | 22 (95.7) | 12 (85.7) | ||
| 1, female | 53 (22.9) | 50 (25.8) | 1 (4.3) | 2 (14.3) | ||
| Tumor quantity, n (%) | 17.305 | 0.002 | ||||
| 0, single | 207 (89.6) | 177 (91.2) | 22 (95.7) | 8 (57.1) | ||
| 1, multiple | 24 (10.4) | 17 (8.8) | 1 (4.3) | 6 (42.9) | ||
| Boundary, n (%) | 4.947 | 0.067 | ||||
| 0, clear | 52 (22.5) | 45 (23.2) | 7 (30.4) | 0 (0) | ||
| 1, unclear | 179 (77.5) | 149 (76.8) | 16 (69.6) | 14 (100) | ||
| Necrosis, n (%) | 2.140 | 0.367 | ||||
| 0, present | 47 (20.3) | 42 (21.6) | 2 (8.7) | 3 (21.4) | ||
| 1, absent | 184 (79.7) | 152 (78.4) | 21 (91.3) | 11 (78.6) | ||
| Arteriovenous fistula, n (%) | 17.465 | 0.008 | ||||
| 0, present | 7 (3) | 4 (2.1) | 0 (0) | 3 (21.4) | ||
| 1, absent | 224 (97) | 190 (97.9) | 23 (100) | 11 (78.6) | ||
| Cirrhosis, n (%) | 0.005 | 0.997 | ||||
| 0, present | 101 (43.7) | 85 (43.8) | 10 (43.5) | 6 (42.9) | ||
| 1, absent | 130 (56.3) | 109 (56.2) | 13 (56.5) | 8 (57.1) | ||
| Peritumoral enhance, n (%) | 14.217 | 0.001 | ||||
| 0, present | 152 (65.8) | 135 (69.6) | 7 (30.4) | 10 (71.4) | ||
| 1, absent | 79 (34.2) | 59 (30.4) | 16 (69.6) | 4 (28.6) | ||
| Satellite nodules, n (%) | 29.967 | <0.001 | ||||
| 0, present | 41 (17.7) | 29 (14.9) | 2 (8.7) | 10 (71.4) | ||
| 1, absent | 190 (82.3) | 165 (85.1) | 21 (91.3) | 4 (28.6) | ||
| HBV, n (%) | 0.052 | 0.974 | ||||
| 0, present | 89 (38.5) | 75 (38.7) | 9 (39.1) | 5 (35.7) | ||
| 1, absent | 142 (61.5) | 119 (61.3) | 14 (60.9) | 9 (64.3) | ||
HCC, hepatocellular carcinoma; MVI, microvascular invasion; PVTT, portal vein tumor thrombosis; HBV, hepatitis B virus.
Multiple logistic regression analysis of tumor thrombus grades in patients with HCC
Variables with a P value <0.05 in the univariate analysis were selected as candidate variables and subsequently analyzed via multiple logistic regression. Multiple logistic regression analysis revealed that tumor diameter, Ki-67 expression level, and peritumoral enhancement were independent risk factors for the formation of tumor thrombus (Table 4). The Ki-67 expression level in the PVTT group and MVI-positive group was higher than that in the MVI-negative group. The area under the curve (AUC) of Ki-67 expression level for predicting tumor thrombus occurrence was 0.713 (95% CI: 0.626–0.800), the cutoff was 17.5%, and the sensitivity and specificity were 61.3% and 75.7%, respectively (P<0.05). The AUC of tumor diameter for predicting tumor thrombus occurrence was 0.753 (95% CI: 0.669–0.837), the cutoff was 4.1 cm, and the sensitivity and specificity were 61.9% and 78.4%, respectively (P<0.05) (Figure 5, Table 5). The AUC of the combined factors was 0.805 (95% CI: 0.723–0.888), with a sensitivity and specificity of 65.5% and 86.5%, respectively (P<0.05).
Table 4
| Parameter | Standard error | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|
| Diameter | 0.055 | 19.1 | 0.239 | 0.132 to 0.346 | <0.001 |
| AFP | 0.000 | 0.634 | 0.000 | −0.001 to 0.001 | 0.426 |
| Ki-67 | 0.016 | 12.007 | 0.057 | 0.025 to 0.089 | 0.001 |
| Tumor quantity | 0.857 | 0.400 | −0.542 | −2.221 to 1.138 | 0.527 |
| Arteriovenous fistula | 0.887 | 3.268 | 1.603 | −0.135 to 3.341 | 0.071 |
| Peritumoral enhance | 0.445 | 5.516 | −1.044 | −1.916 to −0.173 | 0.019 |
| Satellite nodules | 0.768 | 0.209 | 0.351 | −1.154 to 1.856 | 0.648 |
| Gender | 0.705 | 2.501 | 1.115 | −0.267 to 2.496 | 0.114 |
HCC, hepatocellular carcinoma; OR, odds ratio; CI, confidence interval; AFP, alpha fetoprotein.
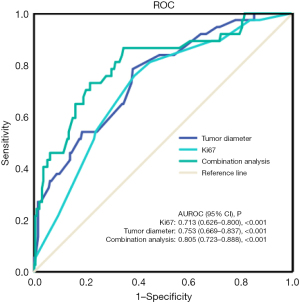
Table 5
| Parameter | AUC | 95% CI | P | Specificity (%) | Sensitivity (%) | Cutoff values |
|---|---|---|---|---|---|---|
| Ki-67 (%) | 0.713 | 0.626–0.800 | <0.001 | 75.7 | 61.3 | 17.5 |
| Tumor diameter (cm) | 0.753 | 0.669–0.837 | <0.001 | 78.4 | 61.9 | 4.1 |
| Combined analysis | 0.805 | 0.723–0.888 | <0.001 | 86.5 | 65.5 | – |
ROC, receiver operating characteristic; HCC, hepatocellular carcinoma; AUC, area under the curve; CI, confidence interval.
Discussion
Tumor thrombus is one of the most important aggressive biological behaviors in patients with HCC and also a critical factor affecting the prognosis of patients with HCC. MVI is an independent risk factor affecting the recurrence free survival time and the overall survival time of liver cancer, and is also an important clinicopathological index significantly related to the invasion and metastasis of liver cancer. If MVI can be predicted before operation, higher requirements should be put forward for the operation, emphasizing the tumor-free operation and radical resection. For postoperative liver cancer with microvascular infiltration, the commonly used and effective method is to transhepatic arterial chemoembolization to further kill residual microcarcinomas and improve postoperative survival rate. The diagnosis of MVI, also as known as microvascular carcinoma thrombosis, mainly depends on pathology (11,12). The incidence of MVI is high even in small HCCs (13,14). MVI tends to occur in small branches of the portal vein in cancer-adjacent tissues and can eventually develop into PVTT. Although PVTT can be well displayed on enhanced CT or MR images as a filling defect in the portal vein with slight enhancement, MVI is difficult to diagnose via imaging examination methods. At present, MVI is mainly diagnosed using a pathology-based 7-point sampling method, but this has certain limitations, and developing a means to predicting MVI in a noninvasive manner has become a goal of recent research. Previous studies (15-18) have shown that the occurrence of MVI is associated with many factors, such as age, cirrhosis, AFP level, tumor number, boundary and diameter of tumor, and abnormal enhancement around the tumor. Ki-67 is a sensitive and highly specific cell marker at proliferative stage, which is an important indicator reflecting the proliferation degree and biological behavior of tumor cells. The higher the positive rate of the marker, the faster the tumor growth, the worse the tissue differentiation, and the worse the prognosis. The expression level of Ki-67 can provide accurate, effective and objective technical means for evaluating the malignant degree of tumor and the efficacy of anti-tumor drugs. However, few studies (19,20) have been conducted concerning the relationship between tumor thrombus and Ki-67 expression level, other tumor indicators, and liver function indicators, and none has been conducted on the correlation between tumor thrombus and Ki-67 expression level. Ki-67, a type of monoclonal antibody, is mainly present in the nucleus, and as it is related to cell mitosis and cell cycle, it can reflect the proliferative ability of tumor cells. A previous study (21) confirmed that the Ki-67 expression level varies according to the degree of malignancy and pathological type of HCC, with the Ki-67 expression level being positively correlated with degree of HCC malignancy and negatively correlated with the prognosis and survival rate.
In this study, univariate analysis showed that there were differences in several aspects, including tumor diameter, AFP expression level, Ki-67 expression level, gender, tumor quantity, arteriovenous fistula, peritumoral enhancement, and satellite nodules, across the three different tumor thrombus grade groups. Of these factors, gender, tumor quantity, and satellite nodules were significantly different, and this result was similar to previous studies (22-24). There was no standard cutoff for tumor diameter used in a study (25), but it is generally accepted that the incidence of tumor thrombus increases with the increase of tumor diameter. The cutoff of tumor diameter in this study was 4.1 cm, which was similar to the results reporting by Liu et al. (22), who considered a tumor diameter greater than >3.9 cm to be an independent risk factor for tumor thrombus classification.
In terms of the correlation between Ki-67 expression level and tumor thrombus, one study (26) found that Ki-67 can synergistically enhance the vascular density in HCC cells to promote the occurrence of tumor thrombus, while another study (27) confirmed that a high expression of Ki-67 is linked to a greater likelihood of HCC recurrence and metastasis. Therefore, there is an urgent need to examine the correlation between Ki-67 expression level and the occurrence of tumor thrombus, as this may provide a key reference for clinical treatment, but no related studies regarding this correlation yet exist.
The results of our study indicated that the Ki-67 expression level of HCC in the MVI-positive and PVTT groups was significantly higher than that in the MVI-negative group. With the increase of Ki-67 expression level, the grade of tumor thrombus increased, revealing that high Ki-67 expression level is more likely to induce invasion and metastasis, leading to a worse prognosis. Logistic analysis further demonstrated that Ki-67 was an independent predictor of tumor thrombus, and its cutoff for predicting tumor thrombus was 17.5%. In addition, the combined diagnostic efficacy of tumor diameter and Ki-67 also was assessed, and the resulting AUC of the combination was 0.805, the sensitivity was 65.5%, and the specificity was 86.5%, indicating that a tumor diameter greater than 4.1 cm and a Ki-67 index value greater than 17.5% are highly suggestive of tumor thrombus. A consideration of these factors can improve the diagnostic accuracy for tumor thrombus after surgery and may constitute an important reference for planning the postoperative treatment of patients.
Peritumoral enhancement can be defined as the abnormal enhancement of the tumor edge in the arterial phase, which manifests as isodense lesions in the delayed and liver parenchyma phase. This area is rich in tumor cells and has a high incidence of tumor thrombus. The results of this study showed that peritumoral enhancement was an independent risk factor for predicting tumor thrombus, which was consistent with previous studies (28,29), and was also associated with the early postoperative recurrence of HCC.
Regarding the correlation between AFP expression level and tumor thrombus, the results of previous studies are generally inconsistent, but most (23,30,31) suggest that AFP expression level is correlated with tumor thrombus classification and is an independent predictor of tumor thrombus occurrence. However, in our study, there was no correlation between AFP expression level and tumor thrombus, and AFP was not an independent risk factor for tumor thrombus. The possible reason for this is that previous studies solely focused on the presence or absence of MVI or different risk levels of MVI, with a PVTT group not being included. As PVTT generally occurs at an advanced stage of tumor, some patients have significantly higher AFP levels, leading to there being no significant correlation between AFP expression level and the occurrence of tumor thrombus. In addition, the small number of patients with PVTT included in this study may also be an influencing factor. Therefore, we will further investigate the correlation between AFP expression level and tumor thrombus.
There are several limitations in this study that should be mentioned. First, a retrospective study design was used. Second, although the sample size of this study was large, the coherence between the three groups was poor. Third, although the radiographic diagnosis of PVTT was clear, the different types of PVTT were not distinguished. Additionally, most of the patients in this study had hepatitis B virus infection or a history of hepatitis B, so our findings may not be applicable to patients with nonviral cirrhosis, such as alcoholic cirrhosis.
Conclusions
Tumor diameter, Ki-67 expression level, and peritumoral enhancement were found to be independent predictors of tumor thrombus. The combination of tumor diameter and Ki-67 expression level demonstrated high diagnostic efficacy for tumor thrombus. We believe these factors can serve as a key index for predicting the formation of tumor thrombus and the prognosis of patients with HCC, thus better informing clinical decision-making.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-983/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-983/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-983/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-983/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First Hospital of Jiaxing (approval No. LS2021-KY-383). The requirement for individual consent was waived by the committee due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145-58. [Crossref] [PubMed]
- Takizawa D, Kakizaki S, Sohara N, et al. Hepatocellular carcinoma with portal vein tumor thrombosis: clinical characteristics, prognosis, and patient survival analysis. Dig Dis Sci 2007;52:3290-5. [Crossref] [PubMed]
- Mähringer-Kunz A, Steinle V, Düber C, et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: The more, the worse? Liver Int 2019;39:324-31. [Crossref] [PubMed]
- Bertuzzo VR, Cescon M, Ravaioli M, et al. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation 2011;91:1279-85. [Crossref] [PubMed]
- Huang ZY, Liang BY, Xiong M, et al. Long-term outcomes of repeat hepatic resection in patients with recurrent hepatocellular carcinoma and analysis of recurrent types and their prognosis: a single-center experience in China. Ann Surg Oncol 2012;19:2515-25. [Crossref] [PubMed]
- Sun X, Yang Z, Mei J, et al. The guiding value of microvascular invasion for treating early recurrent small hepatocellular carcinoma. Int J Hyperthermia 2021;38:931-8. [Crossref] [PubMed]
- Rodríguez-Perálvarez M, Luong TV, Andreana L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 2013;20:325-39. [Crossref] [PubMed]
- Fujita N, Aishima S, Iguchi T, et al. Histologic classification of microscopic portal venous invasion to predict prognosis in hepatocellular carcinoma. Hum Pathol 2011;42:1531-8. [Crossref] [PubMed]
- Ding T, Xu J, Zhang Y, et al. Endothelium-coated tumor clusters are associated with poor prognosis and micrometastasis of hepatocellular carcinoma after resection. Cancer 2011;117:4878-89. [Crossref] [PubMed]
- Huang J, Tian W, Zhang L, et al. Preoperative Prediction Power of Imaging Methods for Microvascular Invasion in Hepatocellular Carcinoma: A Systemic Review and Meta-Analysis. Front Oncol 2020;10:887. [Crossref] [PubMed]
- Zhang EL, Cheng Q, Huang ZY, et al. Revisiting Surgical Strategies for Hepatocellular Carcinoma With Microvascular Invasion. Front Oncol 2021;11:691354. [Crossref] [PubMed]
- Zhang J, Chen G, Zhang P, et al. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS One 2020;15:e0228857. [Crossref] [PubMed]
- Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086-92. [Crossref] [PubMed]
- Du M, Chen L, Zhao J, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer 2014;14:38. [Crossref] [PubMed]
- He Y, Liu F, Mou S, et al. Prognostic analysis of hepatocellular carcinoma on the background of liver cirrhosis via contrast-enhanced ultrasound and pathology. Oncol Lett 2018;15:3746-52. [Crossref] [PubMed]
- Ma X, Wei J, Gu D, et al. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol 2019;29:3595-605. [Crossref] [PubMed]
- Zhu Y, Yang L, Wang M, et al. Preoperative MRI features to predict vessels that encapsulate tumor clusters and microvascular invasion in hepatocellular carcinoma. Eur J Radiol 2023;167:111089. [Crossref] [PubMed]
- Nimitrungtawee N, Inmutto N, Amantakul A, et al. Prediction microvascular invasion of hepatocellular carcinoma based on tumour margin enhancing pattern in multiphase computed tomography images. Pol J Radiol 2023;88:e238-43. [Crossref] [PubMed]
- Schlichtemeier SM, Pang TC, Williams NE, et al. A pre-operative clinical model to predict microvascular invasion and long-term outcome after resection of hepatocellular cancer: The Australian experience. Eur J Surg Oncol 2016;42:1576-83. [Crossref] [PubMed]
- Li HH, Qi LN, Ma L, et al. Effect of KI-67 positive cellular index on prognosis after hepatectomy in Barcelona Clinic Liver Cancer stage A and B hepatocellular carcinoma with microvascular invasion. Onco Targets Ther 2018;11:4747-54. [Crossref] [PubMed]
- Lee JI, Lee JW, Kim JM, et al. Prognosis of hepatocellular carcinoma expressing cytokeratin 19: comparison with other liver cancers. World J Gastroenterol 2012;18:4751-7. [Crossref] [PubMed]
- Liu YQ, Zhao XX. Risk factors for predicting the grade of microvascular invasion in primary hepatocellular carcinoma. Radiologic Practice 2020;35:1453-57.
- Shen J, Wen J, Li C, et al. The prognostic value of microvascular invasion in early-intermediate stage hepatocelluar carcinoma: a propensity score matching analysis. BMC Cancer 2018;18:278. [Crossref] [PubMed]
- Yang Y, Fan W, Gu T, et al. Radiomic Features of Multi-ROI and Multi-Phase MRI for the Prediction of Microvascular Invasion in Solitary Hepatocellular Carcinoma. Front Oncol 2021;11:756216. [Crossref] [PubMed]
- Eguchi S, Takatsuki M, Hidaka M, et al. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg 2010;34:1034-8. [Crossref] [PubMed]
- Zheng R, Zhang X, Liu B, et al. Comparison of non-radiomics imaging features and radiomics models based on contrast-enhanced ultrasound and Gd-EOB-DTPA-enhanced MRI for predicting microvascular invasion in hepatocellular carcinoma within 5 cm. Eur Radiol 2023;33:6462-72. [Crossref] [PubMed]
- Kennedy P. Editorial for "Diagnostic Value of Gd-EOB-DTPA-Enhanced MRI for the Expression of Ki67 and Microvascular Density in Hepatocellular Carcinoma". J Magn Reson Imaging 2020;51:1764-5. [Crossref] [PubMed]
- Zhang X, Wu Z, Peng Y, et al. Correlationship between Ki67, VEGF, and p53 and Hepatocellular Carcinoma Recurrence in Liver Transplant Patients. Biomed Res Int 2021;2021:6651397. [Crossref] [PubMed]
- Du PY, Song JH, Qiao JC, et al. Influencing factors analysis of microvascular invasion in patients with hepatocellular carcinoma. Chinese Journal of Hepatobiliary Surgery 2019;25:26-9.
- Lee S, Kim SH, Lee JE, et al. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 2017;67:526-34. [Crossref] [PubMed]
- Huang Z, Xin JY, Wu LL, et al. Dynamic contrast-enhanced ultrasonography with sonazoid predicts microvascular invasion in early-stage hepatocellular carcinoma. Br J Radiol 2023;96:20230164. [Crossref] [PubMed]


