
PD-1 inhibition combined with intensity-modulated radiotherapy, to better serve patients with retroperitoneal lymph node metastases from gastrointestinal cancer
Highlight box
Key findings
• The effect of programmed death-1 (PD-1) inhibitor combined intensity modulated radiation therapy in patients with retroperitoneal lymph node metastases from gastrointestinal cancer.
What is known and what is new?
• PD-1 inhibitor is useful in gastrointestinal cancer.
• The article suggests the new treatment method of advanced gastrointestinal cancer with retroperitoneal lymph node metastases, and the treatment is safe.
What is the implication, and what should change now?
• The paper supports a new treatment, which can improve patients’ quality of life and be tolerated.
Introduction
Retroperitoneal lymph nodes (RPLNs) are the most widespread site of metastasis for various cancers, including pancreatic, gastric, and hepatic tumors, among others. Metastases to the RPLN generally cause a number of serious clinical symptoms, including pain, abdominal distention, nausea and anorexia, which can affect patient quality of life (1). Traditional surgical resection is very difficult because of the deep location of the RPLN (2,3); maintaining the beneficial effects of chemotherapies in abdominal lymph nodes is also challenging (4-6). Local treatment strategies for RPLN metastasis contain surgical removal, radiofrequency ablation, and radiation treatment (7-9). In current literature, abdominal lymph node may be dissected in advanced esophagogastric junction adenocarcinoma; however, it’s under argument because of different histologies (10). To RPLN metastases of patients with advanced gastric cancer, D2 lymphadenectomy may be sufficient but accompanied with higher morbidity and mortality (11-13). So the extent of lymphadenectomy is still disputed. The proportional gain of radiotherapy (RT) for patients with isolated RPLN metastasis is similar to that for patients with liver or lung metastases, and RT is reported to increase survival time for patients with RPLN metastasis (14). In colorectal cancer patients group with RPLN metastases only, the objective response rate (ORR) and disease control rate (DCR) were 62.5% and 85%, respectively. And in the extra-retroperitoneal metastases group, the ORR and DCR were just 17.9% and 75% (15). Moreover, in the study of locally advanced cervical cancer with RPLN metastases, programmed death-1 (PD-1) inhibitors plus chemoradiotherapy/RT showed effective and safe (16). However, most metastatic gastrointestinal (GI) patients with RPLN metastasis also have extra-retroperitoneal metastases (17), and the data about the combination therapy of PD-1 and intensity-modulated radiation therapy (IMRT), which treated with GI patients with RPLN metastasis, is very rare.
Immunotherapy has recently become the focus of considerable research attention, building a good foundation for the development of GI cancer treatments (18,19). Nevertheless, the efficacy of immune checkpoint blockade therapy is greatly limited by the low immunogenicity and immunosuppressive microenvironment of GI cancer. Application of ionizing radiation to murine tumors has been observed to delay the growth of distant tumors, exhibiting an “abscopal effect”, which can be potentiated by immunostimulatory drugs (20). Further, irradiation might alter the mutation burden and activate new antigen by cancer cells, probably supporting in situ vaccine development and tumor microenvironment reprogramming (21). This phenomenon has prompted oncologists to evaluate the influence of immunotherapy on radiation treatment effects (22); however, these clinical trials to research whether combining IMRT and immunotherapy on GI has meaningful clinical effects are infrequent.
The aim of our study is to assess effect of IMRT combined with PD-1 inhibitors for patients with RPLN metastases who maybe improve survival, control disease and alleviate the local symptoms. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-1011/rc).
Methods
Patients
We performed a single-institution retrospective analysis of GI cancer patients with RPLN metastasis who received PD-1 inhibitors plus IMRT, PD-1 inhibitors alone, or IMRT alone at Ningbo Medical Center Lihuili Hospital between October 2016 and April 2023. The study was retrospective and different treatment methods were based on not only treating physician but also symptoms and disease status of patients. The follow-up time ranged between 4 and 63 months. Patients were followed up by telephone until August 2023. Our study was agreed by the Ethics Committee of Ningbo Medical Center Lihuili Hospital (No. KY2023SL151-01) and all patients or guardians provided informed consent before participating in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The inclusion criteria were as follows: (I) before IMRT or immunotherapy, Eastern Cooperative Oncology Group performance status (ECOG PS), 0–2; (II) pathological diagnosis of stage IV carcinoma except RPLN; (III) measurable RPLN metastasis; (IV) had previously received at least two standard systemic therapeutic regimens. The exclusion criteria were as follows: (I) absence of critical information required for study, such as computed tomography (CT), before and after medication; (II) primary retroperitoneal carcinoma; (III) have a good response to systemic treatment.
Clinical characteristics were from patients’ medical documents, containing age, sex, ECOG PS, hepatitis B virus (HBV), primary tumor site, treatment methods, treatment response and so on.
Radiation technique and medication
In the supine position, patients were secured with abdominal body thermoplastic films. Every patient underwent helical CT (slice thickness, 3 mm) with intravenous contrast. On CT, all involved lymph nodes observed were defined as gross tumor volume (GTV). Clinical target volume (CTV) encompassed the GTV and the localized nodal area, involving 1 cm spreading from GTV in the superior-inferior direction and 0.3 cm spread in anterior-posterior and left-right direction. The scope of expanding the CTV by a margin of 0.7 cm was named the planning target volume (PTV). Radiation was administered to the PTV using IMRT at a dose of 36–60 Gy/8–30 fractions, whose biologically effective dose (BED) was between 96.0 and 43.2 Gy. Plans were considered receivable if the specified dose covered >95% of the PTV and no more than 1 cc received >107% of the prescribed dose. Important normal organs restraints were as follows: ≤50% of the liver received 20 Gy, Dmean ≤30 Gy; stomach Dmax ≤54 Gy; 1 kidney Dmax ≤20 Gy, Dmean ≤18 Gy; ≤10% of the small intestine was to receive 50 Gy; and spinal cord Dmax ≤45 Gy.
PD-1 inhibition treatments were used combined with IMRT if they were initiated within 3–6 weeks after IMRT completion. Treatment was continued until clinical or radiographic disease progression, dose-limiting toxicity, or death (23).
Evaluation of treatment responses
Until disease progression, treatment responses were assessed by total abdominal CT every 2 or 3 months. Tumor response was assessed depending on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (23), containing complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) (23). The ORR was defined as the combination of CR and PR, and the DCR included CR, PR, and SD.
Evaluation of treatment toxicity
Based on the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0) (24), treatment toxicity was followed up continued for 3 months after termination of therapy by the treating physician.
Statistical analysis
Survival curves were analyzed using the Kaplan-Meier method to assess the outcomes. By univariate analysis (UVA) with the log-rank test, the influence of variables potentially impacting progression-free survival (PFS) and overall survival (OS) was evaluated. PFS was defined as the time from starting PD-1 inhibitors or IMRT to occurrence of detectable PD. Systemic PD was defined as disease progression based on RECIST version 1.1. Treatment outcome was assessed as OS, defined as the time from starting IMRT or PD-1 inhibitors until death from final follow-up day or any cause. Cox regression analysis was used to discern the most vital independent prognostic factors and assess hazard ratio (HR) values. The following variables were involved: age, sex, ECOG PS, albumin (ALB), HBV, primary tumor site, treatment methods, and retroperitoneal local tumor response in multivariate analysis. All tests and confidence intervals (CIs) were two-sided, and the significance level of statistical analysis was set at P<0.05. All statistical data analyses were implemented using SPSS 20.0 (IBM Corp, Armonk, NY, USA).
Results
Patients baseline characteristics
Patients (n=98) with histologically confirmed GI cancer and radiographically diagnosed with RPLN metastases were included in the study. Moreover, all included patients had advanced disease that had been previously treated with multiple methods. Patient clinical characteristics are listed in Table 1. The median (range) age of included patients was 62 years (25–84 years); 73 patients were male and 25 were female. A total of 83 patients had ECOG PS 0–1 and 15 had ECOG PS 2. Only local RPLN metastasis was present in 34 patients, whereas 64 patients had RPLN metastasis and distant metastatic. Further, 36 patients had ALB ≤35 g/L and 62 had ALB >35 g/L. On testing for HBV, 69 patients were positive and 29 were negative. Cases included esophageal (n=11), gastric (n=22), colorectal (n=7), hepatocellular (n=43), and pancreatic (n=15) carcinomas.
Table 1
| Characteristic | Patients, n (%) |
|---|---|
| Median age, years [range] | 62 [25–84] |
| Age, years, n (%) | |
| <65 | 62 (63.3) |
| ≥65 | 36 (36.7) |
| Sex, n (%) | |
| Male | 73 (74.5) |
| Female | 25 (25.5) |
| ECOG PS | |
| 0–1 | 83 (84.7) |
| 2 | 15 (15.3) |
| Retroperitoneal local | |
| Yes | 34 (34.7) |
| No | 64 (65.3) |
| ALB (g/L) | |
| ≤35 | 36 (36.7) |
| >35 | 62 (63.3) |
| HBV | |
| Positive | 69 (70.4) |
| Negative | 29 (29.6) |
| Primary tumor location | |
| Esophageal | 11 (11.2) |
| Gastric | 22 (22.4) |
| Colorectal | 7 (7.1) |
| Hepatocellular | 43 (43.9) |
| Pancreatic | 15 (15.3) |
| Therapy | |
| IMRT + Im | 46 (46.9) |
| IMRT | 26 (26.5) |
| Im | 26 (26.5) |
ECOG PS, Eastern Cooperative Oncology Group performance status; ALB, albumin; HBV, hepatitis B virus; IMRT, intensity-modulated radiotherapy; Im, immunotherapy.
Efficacy of treatment and patient survival
Survival analysis indicated that for PD-1 inhibitors plus IMRT team, median (95% CI) PFS was 10.8 months (8.22–13.38), whereas median (95% CI) OS was 12.0 months (10.34–13.66). RPLN variables were evaluated according to local and distant metastatic groups and we found no significant difference in PFS or OS between the two groups. Further, there were no significant differences among patients in the 5 groups classified based on primary tumor location.
UVA showed that therapy method (P=0.032) (Figure 1A) and tumor response (P=0.035) (Figure 1B) were significantly associated with PFS, but not with OS (Table 2). Multivariate analysis did not reveal any obvious correlation with PFS in these patients. At finial follow-up, 70 patients were dead and 28 were alive.
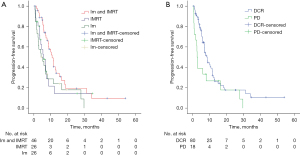
Table 2
| Clinical variable | N | OS | PFS | |||||
|---|---|---|---|---|---|---|---|---|
| Median (months) | 95% CI | Log rank (P value) | Median (months) | 95% CI | Log rank (P value) | |||
| Age, years, n (%) | 0.248 | 0.225 | ||||||
| <65 | 62 | 10.8 | 7.12–14.48 | 7.8 | 6.07–9.53 | |||
| ≥65 | 36 | 10.8 | 6.68–14.92 | 7.0 | 4.69–10.31 | |||
| Sex, n (%) | 0.863 | 0.561 | ||||||
| Male | 73 | 10.8 | 8.29–13.31 | 8.0 | 6.33–9.67 | |||
| Female | 25 | 10.0 | 5.31–14.69 | 7.2 | 4.88–9.52 | |||
| ECOG PS | 0.438 | 0.642 | ||||||
| 0–1 | 83 | 10.8 | 8.01–13.59 | 7.5 | 5.92–9.08 | |||
| 2 | 15 | 10.0 | 0.79–19.21 | 8.9 | 0.00–19.02 | |||
| Retroperitoneal local | 0.610 | 0.132 | ||||||
| Yes | 34 | 12.0 | 9.76–14.24 | 10.0 | 7.71–12.29 | |||
| No | 64 | 9.3 | 6.68–11.92 | 6.8 | 5.40–8.20 | |||
| ALB (g) | 0.518 | 0.681 | ||||||
| ≤35 | 70 | 9.8 | 6.68–12.92 | 7.1 | 5.18–9.03 | |||
| >35 | 28 | 10.8 | 7.95–13.66 | 10.8 | 4.99–16.61 | |||
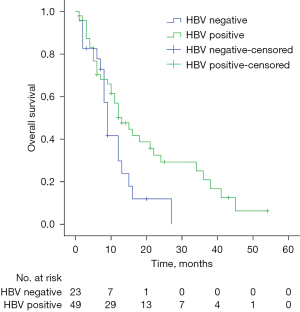
| HBV | 0.316 | 0.908 | ||||||
| Positive | 69 | 11.1 | 8.93–13.27 | 7.1 | 4.56–9.65 | |||
| Negative | 29 | 9.0 | 7.46–10.54 | 8.0 | 5.40–10.61 | |||
| Primary tumor location | 0.822 | 0.397 | ||||||
| Esophageal | 11 | 9.5 | 1.43–17.57 | 7.0 | 0.81–13.19 | |||
| Gastric | 22 | 11.5 | 7.42–15.58 | 10.0 | 6.35–13.65 | |||
| Colorectal | 7 | 8.6 | 1.93–15.27 | 6.9 | 3.82–9.98 | |||
| Hepatocellular | 43 | 10.8 | 7.24–14.36 | 8.7 | 6.39–11.01 | |||
| Pancreatic | 15 | 6.0 | 0.00–15.71 | 4.2 | 0.13–8.27 | |||
| Therapy | 0.158 | 0.032 | ||||||
| IMRT + Im | 46 | 12.0 | 10.34–13.66 | 10.8 | 8.22–13.38 | |||
| IMRT | 26 | 8.1 | 3.98–12.22 | 5.7 | 2.62–8.78 | |||
| Im | 26 | 8.0 | 3.63–12.37 | 4.3 | 0.70–7.90 | |||
| Response | 0.471 | 0.035 | ||||||
| DCR | 80 | 10.8 | 8.61–12.99 | 8.5 | 6.66–10.34 | |||
| PD | 18 | 3.3 | 1.22–5.38 | 2.7 | 2.15–3.25 | |||
OS, overall survival; PFS, progression-free survival; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; ALB, albumin; HBV, hepatitis B virus; IMRT, intensity-modulated radiotherapy; Im, immunotherapy; DCR, disease control rate; PD, progressive disease.
Treatment responses of RPLN lesions are presented in Table 3. In the PD-1 inhibitors plus IMRT group, 1 patient (2.2%) achieved CR, 30 (65.2%) had PR, and 14 (30.4%) had SD. In the IMRT only group, 12 (46.2%) and 13 (50.0%) of 26 patients had PR and SD, respectively; no patients achieved CR. Further, the PR (7.7%) and PD (61.5%) rates were inferior in the PD-1 inhibitors alone group. Moreover ORR (67.4%) and DCR (97.8%) were significantly higher in the PD-1 inhibitors plus IMRT group than those in the other two groups.
Table 3
| Response | No. patients who received, n (%) | ||
|---|---|---|---|
| IMRT + Im (n=46) | IMRT (n=26) | Im (n=26) | |
| CR | 1 (2.2) | 0 | 0 |
| PR | 30 (65.2) | 12 (46.2) | 2 (7.7) |
| SD | 14 (30.4) | 13 (50.0) | 8 (30.8) |
| PD | 1 (2.2) | 1 (3.8) | 16 (61.5) |
| ORR | 31 (67.4) | 12 (46.2) | 2 (7.7) |
| DCR | 45 (97.8) | 25 (96.2) | 10 (38.5) |
RPLN, retroperitoneal lymph nodes; IMRT, intensity-modulated radiotherapy; Im, immunotherapy; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
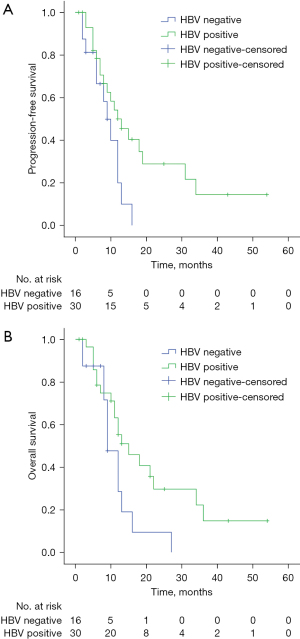
UVA of data from the PD-1 inhibitors combined with IMRT and PD-1 inhibitors only groups suggested that HBV status was clearly associated with OS (P=0.041) (Figure 2), but not with PFS; OS was not associated with HBV status in these groups on multivariate analysis. However, HBV status was associated with PFS (P=0.041) (Figure 3A) and OS (P=0.049) (Figure 3B) on UVA of the inhibitors plus IMRT group, but not with PFS or OS on multivariate analysis.
Toxicity and safety
PD-1 inhibitors were well withstood, with just 2 patients experiencing grade 3 hypothyroidism (2.8%). During RT, 6 patients (8.3%) developed acute GI adverse reactions such as bloating and nausea, including 2 patients with grade 3 toxicity. No cases of grade 4 and/or higher toxicity was detected in patients treated with IMRT or PD-1 inhibitors.
Discussion
Treatment of patients with RPLN metastasis of GI cancer is still an important clinical challenge. Treatment modalities to control RPLN generally include surgical resection, RT, and chemotherapy, yet the validity of these therapeutic modalities remains weak and the prognosis of patients with RPLN metastasis continues to be poor. There is no consensus on the appropriate treatment strategy. The goal of our research was to explore the safeness and usefulness of PD-1 inhibitors plus IMRT for RPLN in patients with GI cancer.
The biological response of tumor cells to RT involves damage, repair, and apoptosis or necrosis, causing the activation or inhibition of signal transduction pathways involved in DNA repair, metabolism, and cell cycle arrest (25). RNA-seq analysis has shown that a few of the genes modulating cancer proliferation, invasion, and immune response may be up- or down-regulated in tumor tissues after RT. Further, the expression levels of numerous immune-related genes change in response to RT. The tumor immune microenvironment includes inflammatory factors, infiltrating immune cells, and stromal cells, and could be reprogrammed by radiation (26). Expression of CXXL10, CXCL16, interferons (IFNs), and IFN receptors can be affected by irradiation in an experimental animal prototype (27,28). Zhou et al. showed that the diversity of the T-cell receptor repertoire and programmed death ligand 1 (PD-L1) expression increased obviously in the microenvironment following stereotactic body radiotherapy (SBRT), with ‘immune response gene’ the richest terminology on Gene Ontology analysis (29). Increased PD-L1 expression can improve the function of PD-1 inhibitors. Furthermore, Theelen et al. demonstrated a positive effect of pembrolizumab after SBRT on tumor response in PD-L1-negative patients with advanced non-small cell lung cancer (NSCLC), who had significantly improved PFS and OS. In that study, the ORR of the pembrolizumab combined SBRT arm was double that of the pembrolizumab alone arm. Further, SBRT prior to pembrolizumab was well-tolerated. The authors concluded that RT possibly activates non-inflamed NSCLC toward a more inflamed tumor microenvironment (30).
Immunotherapy has had profound effects on cancer treatments (31). A randomized phase III study showed that PD-1 inhibitors are useful following neoadjuvant chemoradiotherapy (CRT) and surgery in patients with esophageal/gastroesophageal junction cancers who had resected stage II or III cancer and were assigned in a 2:1 fashion to receive nivolumab or placebo with the primary finishing line being disease-free survival. Compared to the placebo team whose median disease-free survival was 11.0 months, nivolumab extended median disease-free survival to 22.4 months. Further, nivolumab was shown to reduce the risk of distant recurrence or death by 26% and increase median metastasis free survival time to 10.7 months compared to the placebo group; 71 patients experienced grade 3 or 4 adverse events related to the nivolumab (32). The phase 2 PACIFIC-6 trial showed that median PFS was 10.9 months, whereas 12-month PFS and OS rates were 49.6% and 84.1%, respectively. Only 5 of 117 (4.3%) patients had grade 3 or 4 possibly related adverse events within 6 months of starting treatment. It has previously been demonstrated that IMRT combined with immunotherapy was well-tolerated in a real-life environment, with safety observations aligned with the profile of durvalumab administered after CRT in patients with unresectable, stage III NSCLC (33-35). Furthermore, PACIFIC-R was an international, retrospective study, which reported median PFS of 21.7 months. In the real world, the PFS of patients received concurrent CRT was longer than that of those who were treated by sequential CRT (median, 23.7 vs. 19.3 months, P>0.05) and among patients with PD-L1 expression ≥1% vs. <1% (22.4 vs. 15.6 months, P>0.05). These favorable real-world PFS outcomes were found in spite of PD-L1 expression and time of immunotherapy plus CRT (36). In our study, the median PFS of patients with RPLN metastases from GI cancer treated with IMRT plus PD-1 inhibitor reached 10.8 months (P=0.032), which was longer than that of other treatments.
Several retrospective studies have indicated the benefit of radiation treatment in patients with isolated RPLN in recent years (37,38). Shu et al. stated the results of 68 patients who were cured between 2009 and 2018 with IMRT (dose, 50–50.4 Gy/25–28 fractions) who had RPLN metastases. In the isolated RPLN metastases group, CR was detected in 5 patients (12.5%), PR was achieved in 20 patients (50%), and 9 patients (22.5%) had SD. The median OS values were 59.4 in the isolated RPLN metastases group and 19 months in the extra-retroperitoneal metastases group (15). Similarly, the ORR to PD-1 inhibitors plus IMRT, IMRT only, and PD-1 only reached 67.4%, 46.2%, and 7.7%, respectively, and the DCR values for the 3 groups were 97.8%, 96.2%, and 38.5%, respectively, in our current retrospective study.
The results of subgroup analyses of patients receiving IMRT plus PD-1 inhibitors and PD-1 inhibitor only were also consistent with those of previous studies (39,40). An OS benefit was observed in HBV-positive nivolumab-treated patients (HR =0.77) (39). A similar finding was observed in an HBV-positive hepatocellular carcinoma subgroup in the KEYNOTE-240 trial of pembrolizumab, with HR values for PFS of 0.70 (0.44–1.13) and for OS of 0.57 (0.36–0.94) (40). PD-1 inhibitors have significantly improved the survival of illnesses with advanced HBV-positive liver cancer, but the risk of HBV reactivation from these antitumor drugs remains unclear. Lei et al. found that the PD-1 inhibitor therapy group (HR =1.41, P=0.05) represented a separate risk factor for HBV reactivation. In the HBV non-reactivation group, patients’ PFS and OS were significantly prolonged (P<0.001 and P=0.001) compared to the HBV reactivation group (41). Further studies are needed to refine the definition of HBV reactivation to discover risk factors, and to prevent and manage HBV reactivation in HBsAg-positive patients with hepatocellular carcinoma receiving immunotherapy.
Limitations
The current study has some limitations; for example, RPLN metastases were not confirmed pathologically. The effect of RT dose, fractionation, and different PD-1 inhibitors on antitumor immune responses is unknown. And needing more reliable diagnostic method to stratify RPLN patients can be helpful to choose more suitable treatment for every patient, such as dynamic contrast-enhanced magnetic resonance imaging parameters (42). Moreover, our study was retrospective in nature. Deviation of analysis results may have occurred due to the small sample size, variation in patient baseline characteristics, different treatment regimens, unknown microsatellite instability status, and unknown PD-L1 expression status. UVA on small samples incurs a great risk of type II errors. Further study is needed to determine whether the RT dose and time used in this clinical research were optimal, in regard to the immune-modulating potentiality of IMRT in combination with PD-1 inhibitions in patients with RPLN metastases from GI cancer.
Conclusions
The present retrospective study of PD-1 inhibitors combined with IMRT to treat RPLN metastases from GI cancer indicated that the combination was safe. Further, the efficacy of patients provided us with a feasible therapy option besides PD-1 inhibitors or IMRT alone. Large prospective randomized clinical studies are needed to determine the safety and efficacy of combining PD-1 inhibitors and IMRT.
Acknowledgments
Funding: The study was supported by the scientific research project of innovative application of professional ability of clinical professionals: multi-omics exploration of biomarkers for predicting the efficacy of neoadjuvant chemoradiotherapy in locally advanced pancreatic cancer.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-1011/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-1011/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-1011/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-1011/coif). M.D.C. receives grants from ViewRay, Novocure, StratPharma, and payment or honoraria for lectures from ViewRay, Sirtex, IBA, and consulting fees from ViewRay; and serves on board of Proton Collaborative Group, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our study was agreed by the Ethics Committee of Ningbo Medical Center Lihuili Hospital (No. KY2023SL151-01) and all patients or guardians provided informed consent before participating in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hino H, Kagawa H, Kinugasa Y, et al. Long-term survival with surgery for metachronous retroperitoneal lymph node and pancreatic metastases after curative resection of rectal cancer: a case report. Surg Case Rep 2016;2:49. [Crossref] [PubMed]
- Gagnière J, Dupré A, Chabaud S, et al. Retroperitoneal nodal metastases from colorectal cancer: Curable metastases with radical retroperitoneal lymphadenectomy in selected patients. Eur J Surg Oncol 2015;41:731-7. [Crossref] [PubMed]
- Persson J, Geppert B, Lönnerfors C, et al. Description of a reproducible anatomically based surgical algorithm for detection of pelvic sentinel lymph nodes in endometrial cancer. Gynecol Oncol 2017;147:120-5. [Crossref] [PubMed]
- Shindoh J, Kaseb A, Vauthey JN. Surgical strategy for liver cancers in the era of effective chemotherapy. Liver Cancer 2013;2:47-54. [Crossref] [PubMed]
- Thomas MB. Systemic and targeted therapy for biliary tract tumors and primary liver tumors. Surg Oncol Clin N Am 2014;23:369-81. [Crossref] [PubMed]
- Suh BJ. A Case of Advanced Gastric Cancer with Para-Aortic Lymph Node Metastasis Treated with Preoperative FOLFOX Chemotherapy Followed by Radical Subtotal Gastrectomy and D2 Lymph Node Dissection. Case Rep Oncol 2017;10:182-91. [Crossref] [PubMed]
- Van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 2006;42:2212-21. [Crossref] [PubMed]
- Dhir M, Sasson AR. Surgical Management of Liver Metastases From Colorectal Cancer. J Oncol Pract 2016;12:33-9. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Hutchings H, Okereke IC. The different routes of lymph node metastases in esophageal cancer and its significance. J Thorac Dis 2023;15:5873-6. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [PubMed]
- Galizia G, Lieto E, De Vita F, et al. Modified versus standard D2 lymphadenectomy in total gastrectomy for nonjunctional gastric carcinoma with lymph node metastasis. Surgery 2015;157:285-96. [Crossref] [PubMed]
- de Jong MHS, Gisbertz SS, van Berge Henegouwen MI, et al. Lymph node metastases rate of locoregional and non-locoregional lymph node stations in gastric cancer. J Gastrointest Oncol 2022;13:1605-15. [Crossref] [PubMed]
- Yeo SG, Kim DY, Kim TH, et al. Curative chemoradiotherapy for isolated retroperitoneal lymph node recurrence of colorectal cancer. Radiother Oncol 2010;97:307-11. [Crossref] [PubMed]
- Shu P, Ouyang G, Wang F, et al. The Role of Radiotherapy in the Treatment of Retroperitoneal Lymph Node Metastases from Colorectal Cancer. Cancer Manag Res 2020;12:8913-21. [Crossref] [PubMed]
- Liu C, Ran X, Wang Z, et al. Efficacy and safety of PD-1 inhibitor combined with concurrent chemoradiotherapy in locally advanced cervical cancer with pelvic and/or para-aortic lymph node metastases: a retrospective cohort study. Chin Clin Oncol 2023;12:38. [Crossref] [PubMed]
- Hashimoto M, Komatsu H, Naruse Y, et al. Resection of paraaortic lymph node metastasis of colon cancer with graft replacement. Hepatogastroenterology 2003;50:709-10. [PubMed]
- Zou J, Huang P, Ge N, et al. Anti-PD-1 antibodies plus lenvatinib in patients with unresectable hepatocellular carcinoma who progressed on lenvatinib: a retrospective cohort study of real-world patients. J Gastrointest Oncol 2022;13:1898-906. [Crossref] [PubMed]
- Madan A, Uronis HE, Strickler JH. A narrative review of the evolving role of immunotherapy in the management of esophageal and gastric cancer. J Gastrointest Oncol 2022;13:2007-19. [Crossref] [PubMed]
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. [Crossref] [PubMed]
- Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin 2017;67:65-85. [Crossref] [PubMed]
- Hino C, Lee EW, Yang GY. Harnessing the abscopal effect for gastrointestinal malignancies in the era of immunotherapy. J Gastrointest Oncol 2023;14:1613-25. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr 2021;112:90-2. (Engl Ed). [Crossref] [PubMed]
- Broustas CG, Xu Y, Harken AD, et al. Comparison of gene expression response to neutron and x-ray irradiation using mouse blood. BMC Genomics 2017;18:2. [Crossref] [PubMed]
- Barker HE, Paget JT, Khan AA, et al. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015;15:409-25. [Crossref] [PubMed]
- Rentoft M, Coates PJ, Loljung L, et al. Expression of CXCL10 is associated with response to radiotherapy and overall survival in squamous cell carcinoma of the tongue. Tumour Biol 2014;35:4191-8. [Crossref] [PubMed]
- Wang X, Schoenhals JE, Li A, et al. Suppression of Type I IFN Signaling in Tumors Mediates Resistance to Anti-PD-1 Treatment That Can Be Overcome by Radiotherapy. Cancer Res 2017;77:839-50. [Crossref] [PubMed]
- Zhou P, Chen D, Zhu B, et al. Stereotactic Body Radiotherapy Is Effective in Modifying the Tumor Genome and Tumor Immune Microenvironment in Non-Small Cell Lung Cancer or Lung Metastatic Carcinoma. Front Immunol 2021;11:594212. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. [Crossref] [PubMed]
- Pointer KB, Pitroda SP, Weichselbaum RR. Radiotherapy and immunotherapy: open questions and future strategies. Trends Cancer 2022;8:9-20. [Crossref] [PubMed]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. [Crossref] [PubMed]
- Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol 2021;16:860-7. [Crossref] [PubMed]
- Garassino MC, Mazieres J, Reck M, et al. Durvalumab After Sequential Chemoradiotherapy in Stage III, Unresectable NSCLC: The Phase 2 PACIFIC-6 Trial. J Thorac Oncol 2022;17:1415-27. [Crossref] [PubMed]
- Girard N, Bar J, Garrido P, et al. Treatment Characteristics and Real-World Progression-Free Survival in Patients With Unresectable Stage III NSCLC Who Received Durvalumab After Chemoradiotherapy: Findings From the PACIFIC-R Study. J Thorac Oncol 2023;18:181-93. [Crossref] [PubMed]
- Kim MS, Cho CK, Yang KM, et al. Stereotactic body radiotherapy for isolated paraaortic lymph node recurrence from colorectal cancer. World J Gastroenterol 2009;15:6091-5. [Crossref] [PubMed]
- Johnson B, Jin Z, Haddock MG, et al. A Curative-Intent Trimodality Approach for Isolated Abdominal Nodal Metastases in Metastatic Colorectal Cancer: Update of a Single-Institutional Experience. Oncologist 2018;23:679-85. [Crossref] [PubMed]
- Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022;23:77-90. [Crossref] [PubMed]
- Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2020;38:193-202. [Crossref] [PubMed]
- Lei J, Yan T, Zhang L, et al. Comparison of hepatitis B virus reactivation in hepatocellular carcinoma patients who received tyrosine kinase inhibitor alone or together with programmed cell death protein-1 inhibitors. Hepatol Int 2023;17:281-90. [Crossref] [PubMed]
- Ciolina M, Caruso D, De Santis D, et al. Dynamic contrast-enhanced magnetic resonance imaging in locally advanced rectal cancer: role of perfusion parameters in the assessment of response to treatment. Radiol Med 2019;124:331-8. [Crossref] [PubMed]


