
Postoperative superior anastomotic leakage classification and treatment strategy for postoperative esophagogastric junction cancer
Highlight box
Key findings
• After surgery for adenocarcinoma of the esophagogastric junction, suitable treatment measures should be adopted according to the type of superior anastomotic leakage that occurs. Types III and IV superior anastomotic leakages are associated with higher mortality and require greater attention from surgeons.
What is known and what is new?
• This study collected clinical data of 57 patients with anastomotic leakage, and innovatively proposed the classification of anastomotic leakage and corresponding treatment strategy.
• The results of this study can provide theoretical guidance for surgeons to diagnose and treat patients with substantial anastomotic leakage after gastric cancer surgery.
What is the implication, and what should change now?
• Due to the small number of cases included and the single-center design, this study may involve a certain degree of selection bias and other limitations. In order to further verify our findings, additional research with large samples and a multicenter design is needed.
Introduction
The epidemiological data from Europe, the United States, and Japan indicate that the incidence of esophagogastric junction (EGJ) squamous cell carcinoma has decreased year by year over the past 30 years, while that of adenocarcinoma of the EGJ (AEG) has increased gradually (1,2). The clinical experience of experts in China suggests that the incidence of AEG has also increased sharply over the past 10 years (3). Common surgical approaches for AEG include transabdominal, transdiaphragmatic hiatus, and left thoracoabdominal operation, among others. Postoperative reconstruction methods for superior anastomosis include esophagojejunostomy and esophagogastrostomy. Due to the superior position of the anastomosis compared with sites of gastrojejunal anastomosis and jejunojejunal anastomosis, postoperative anastomotic leakage often occurs, and the anastomosis is often located in the posterior mediastinum. Once the anastomotic leakage occurs, the posterior mediastinum and the left thoracic cavity are often seriously infected, which further impairs respiratory and circulatory function, heightening the danger of the disease course. A case fatality rate of 15% to 50% has been reported for anastomoses of a high position (4-6). The literature suggests that there are several types of anastomotic leakage that can occur after esophageal cancer surgery, with researchers often discussing the classification of anastomotic leakage after surgical treatment for esophageal cancer (7). Based on our clinical experience at the Department of General Surgery of the Affiliated Cancer Hospital of Zhengzhou University in handling these patients, both transferred within our hospital and from other hospitals, we propose a classification of high-position anastomotic leakage after surgery for AEG and discuss the corresponding treatment methods. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-968/rc).
Methods
Patient data
This study was a retrospective clinical study and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The data of 1,652 AEG patients in the Department of General Surgery of the Affiliated Cancer Hospital of Zhengzhou University from January 2017 to March 2019 were statistically analyzed. Anastomotic leakage occurred in 30 cases (2.17%) and 27 cases were transferred from other hospitals, 57 cases in total. The study was approved by the ethics board of the Affiliated Cancer Hospital of Zhengzhou University (No. 2021-132-006) and individual consent for this retrospective analysis was waived.
The analysis included treated patients classified according to the Siewert’s classification (8). In patients classified as AEG I, upper stomach resection with esophageal resection and esophagogastrostomy in the chest with D2+ lymphadenectomy was performed, while in the AEG II and III group, gastrectomy with D2 lymphadenectomy was undertaken. Intraoperatively, anastomosis was performed in all patients after contrast swallow examination and confirmation of its integrity in endoscopic examination. Anastomosis was performed using a stapler in all patients. In the postoperative period, confirmation of anastomotic leakage was obtained after diagnostic tests such as upper gastrointestinal contrast swallow study contrast-enhanced computed tomography (CT), and endoscopy assessing the integrity of the anastomosis.
Esophagogastric anastomotic leakage was defined according to the proposed definition by the Esophagectomy Complications Consensus Group (ECCG) and was defined as a full-thickness gastrointestinal defect involving the esophagus anastomosis, staple line, or conduit, irrespective of presentation or method identification (9).
We categorized patients into four types according to the size and risk of anastomotic leakage as follows:
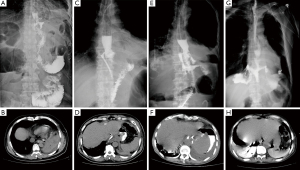
- Type I (subclinical leakage) was defined as anastomotic leakage found in routine upper gastrointestinal angiography after surgery, usually with a diameter of less than 2 mm and no clinical symptoms (Figure 1A,1B).
- Type II, with a diameter of 2–10 mm, was clinically defined as postoperative fever, turbidity of drainage fluid, anastomotic leakage confirmed by imaging, smooth irrigation and drainage of the drainage tube, or CT review after puncture drainage showing no obvious fluid collection area (Figure 1C,1D).
- Type III leakage with a diameter of >10 mm without necrosis of conduit confirmed by endoscopic examination (Figure 1E,1F). This type is characterized by postoperative chills, high fever, turbidity of the drainage fluid, or accompanying odor, with CT review after puncture drainage still showing obvious areas of fluid, and stable hemodynamics.
- Type IV with a diameter usually >20 mm with necrosis of the conduit confirmed by endoscopy and CT (Figure 1G,1H). This type is clinically characterized by postoperative shivering, high fever, turbidity of the drainage fluid, or accompanying malodorous fluid. CT reexamination after puncture drainage still shows obvious areas of fluid with accompanying hemodynamic instability.
Inclusion and exclusion criteria
The patient inclusion criteria were as follows: (I) surgical R0 resection; (II) patients with confirmed intraoperatively integrity of the esophagogastric or esophagointestinal anastomosis; and (III) postoperative esophagojejunostomy leakage or esophagogastrostomy anastomosis leakage. The patient exclusion criteria were as follows: palliative resection and postoperative jejunal or duodenal residual leakage.
Treatment
- For type I leakage, treatment included diet prohibition, gastrointestinal decompression, enteral nutrition support with a naso-enteral nutrition tube or combination with intravenous high-nutrition support, as well as liquid feeding after upper digestive tract angiography for two reexaminations to rule out esophagojejunal or esophagogastric anastomotic leakage.
- For type II leakage, treatment included interventional or color ultrasound puncture drainage, in which the drainage tube was placed next to the leakage of the esophagojejunal anastomosis; otherwise, nasal irrigation drainage tube was placed under intervention, and the double-cannula drainage was replaced. A stent or titanium clip was placed under the endoscope to close the leakage, or biological glue was used to seal the leakage. Then, the patients were treated with diet prohibition, gastrointestinal decompression, enteral nutrition support through naso-intestinal nutrition tube or combined with intravenous hypernutrition support, and broad-spectrum antibiotics.
- For type III leakage, treatment would be surgical intervention: for those with early infection and no severe adhesion, the leakage was repaired or mechanical anastomosis of the esophagojejunum or esophagojejunum remnant stomach was reperformed, and tube leakage and decompression were completed in the jejunum loop of the replacement stomach or remnant stomach. For those severe abdominal infections, the fluid around the anastomosis and infected tissue was removed, and tube leakage and decompression were completed in the replacement gastrojejunal loop or remnant stomach.
- For type IV leakage, hemodynamic instability was corrected as soon as possible, and emergency operation was performed. Necrotic tissue including in the esophagus, jejunum, or gastric remnant was removed thoroughly; surrounding fluid and necrotic infected tissue were removed; mechanical anastomosis of the esophageal jejunum or gastric remnant was performed again, and tube leakage and decompression were completed in the gastric jejunum loop or gastric remnant.
Statistical analysis
SPSS 22.0 statistical software (IBM Corp., Armonk, NY, USA) was used for data processing, and measurement data are expressed as mean ± standard deviation (SD). Comparison between groups was conducted using the independent-samples t-test for variables with a normal distribution. The Mann-Whitney test was used if the distribution did not conform to normal. Count data are expressed as the count and percentage, and the comparison between the two groups was conducted using the Fisher exact probability method. A P value <0.05 indicated a statistically significant difference.
Results
Comparison of general data between the two groups
Fifty-seven patients of esophagojejunostomy leakage were enrolled to the study, five of whom with esophagogastrostomy leakage, and none with jejunojejunostomy leakage. Among these, 50 were males and seven were females, with an age range of 33–76 years and an average age of 63.8 years.
In terms of age, sex, preoperative albumin level, body mass index (BMI), first operation time, first operation blood loss, neoadjuvant therapy, tumor diameter, pathological type, tumor-node-metastasis (TNM) stage, and surgical method, there were no significant differences between types I and II leakages and types III and IV leakages (P>0.05). Types I and II leakages were more common in patients undergoing abdominal approach surgery and with no complications before surgery, while types III and IV leakages were significantly more common in patients treated by the combined phrenic hiatus and the thoracoabdominal approach or by open surgery and those with complications before surgery (Table 1).
Table 1
| Items | Types I and II (n=30) | Types III and IV (n=27) | χ2/t | P |
|---|---|---|---|---|
| Age (years) | 60.0±8.3 | 61.1±11.7 | −0.382 | 0.704 |
| Gender | 0.283 | 0.594 | ||
| Male | 24 | 20 | ||
| Female | 6 | 7 | ||
| Preoperative albumin (g/L) | 0.236 | 0.627 | ||
| <30 | 3 | 1 | ||
| ≥30 | 27 | 26 | ||
| BMI (kg/m2) | 24 | 24.3 | 0.4 | 0.691 |
| First operation time (min) | 146.0±18.95 | 141.4±21.2 | 0.828 | 0.411 |
| Initial operation bleeding (mL) | 241.7±170.2 | 254.5±173.8 | −0.267 | 0.79 |
| Site of initial operation | 7.721 | 0.005 | ||
| Our hospital | 24 | 12 | ||
| Local hospital | 6 | 15 | ||
| Operative approach | 9.372 | 0.002 | ||
| Transabdominal approach | 28 | 16 | ||
| Diaphragmatic hiatus/thoracoabdominal approach | 2 | 11 | ||
| Operative procedure | 0.351 | 0.554 | ||
| Total gastrectomy | 28 | 24 | ||
| Proximal gastrectomy | 2 | 3 | ||
| Neoadjuvant chemotherapy | 1.201 | 0.273 | ||
| Yes | 8 | 4 | ||
| No | 22 | 23 | ||
| Mean tumor diameter (cm) | 4.6 | 5.1 | −0.895 | 0.375 |
| Pathological pattern | 1.267 | 0.26 | ||
| Differentiated | 8 | 11 | ||
| Undifferentiated | 22 | 16 | ||
| TNM stage | 0.31 | 0.857 | ||
| I | 2 | 1 | ||
| II | 12 | 12 | ||
| III | 16 | 14 | ||
| Complication | 6.318 | 0.012 | ||
| Yes | 10 | 18 | ||
| No | 20 | 9 | ||
| Type of operation | 4.053 | 0.044 | ||
| Open operation | 12 | 18 | ||
| Laparoscopic surgery | 18 | 9 |
Data are shown as mean ± SD or n. BMI, body mass index; TNM, tumor-node-metastasis; SD, standard deviation.
Comparison of postoperative convalescence-related clinical indexes between the leakage type groups
The average time for diagnosis of types I and II leakages was 4.8±1.4 days after the first surgery, while the average time for diagnosis of types III and IV leakages was 6.3±1.5 days after the first surgery, with the difference in time being statistically significant (P<0.05). The time to start oral feeding (56.5±17.5 days) and the length of hospital stay after the first operation (67.1±17.3 days) for types III and IV leakages were significantly longer than those for types I and II leakages (25.2±5.2 and 31.2±5.19 days, respectively; P<0.05). Compared with that of patient’s with types I and II leakages, the disease of patients with types III and IV leakages was significantly more serious, and more often included peritonitis, bacteremia, cardiac insufficiency, pneumonia, other complications, the need for more than two doses of antibiotics, and combination treatment with antifungal drugs. It was found that the mortality rate within 90 days after surgery of patients with types III and IV leakages was 14.8%, while there were no deaths among patients with types I and II leakages, and this difference in mortality was statistically significant (Table 2).
Table 2
| Items | Types I and II (n=30) | Types III and IV (n=27) | χ2/t | P |
|---|---|---|---|---|
| Time to confirm anastomotic leakage (days) | 4.8±1.4 | 6.3±1.5 | −3.618 | <0.001 |
| Length of stay after first operation (days) | 31.2±5.19 | 67.1±17.3 | −10.828 | <0.001 |
| Time to start eating after surgery (days) | 25.2±5.2 | 56.5±17.5 | −9.278 | <0.001 |
| Other postoperative complications | 23.945 | <0.001 | ||
| Yes | 5 | 22 | ||
| No | 25 | 5 | ||
| Antibiotic use | 21.493 | <0.001 | ||
| Single | 24 | 5 | ||
| Combined | 6 | 22 | ||
| Death | 0 (0.0) | 4 (14.8) | 4.780 | 0.029 |
Data are shown as mean ± SD, n, or n (%). SD, standard deviation.
Discussion
With the progress of surgical technology, especially the gradual development of minimally invasive surgery, the occurrence of anastomotic leakage after surgery for AEG is also gradually declining, but the occurrence of leakage still cannot be avoided in clinical practice. When the anastomosis is superior, leakage often leads to serious posterior mediastinal, thoracic, and abdominal infections, which greatly affects the short-term and long-term prognosis of these patients. Ma et al. (8) reported that after radical gastrectomy, the incidence of anastomotic leakage was 6.3%, and the mortality related to anastomotic leakage reached 9%. Nagasako et al. (9) found that the incidence of anastomotic complications in laparoscopic gastric cancer was 9.3%, and compared with patients without anastomotic complications, patients with anastomotic complications had a lower 5-year survival rate. Tsou et al. (10) reported that the incidence of postoperative anastomotic leakage for gastric cancer was 2.7%, and the mortality related to anastomotic leakage was as high as 21.1%. Li et al. (11) and Yoo et al. (12) both reported that in patients with gastric cancer, the overall survival time of patients with anastomotic leakage was significantly lower than that of patients without anastomotic leakage. Based on the presented analysis, we can observe the complexity of the problem posed by a fistula in esophagogastric anastomosis after gastrectomy or upper gastric resection. The treatment strategy for fistulas is challenging due to the lack of recommendations and proposed standards in their management. There is no doubt that the decision to initiate treatment depends on the experience of the medical center. The success of our approach is influenced by considering multiple factors, among which we should take into account the fistula location, size, vascularization in the anastomosis and conduit area, and the time of its occurrence. The use of diagnostic methods such as angiography, tomography, and endoscopy allow for the assessment of the fistula, while classification helps in determining the treatment strategy.
There is a lack of clear classification of postoperative anastomotic leakage for gastric cancer in the literature, with most studies referring to the classification of postoperative anastomotic leakage for esophageal cancer. There are many types of anastomotic leakage after operation for esophageal cancer, with two types, three types, and four types being described according to different observation angles, providing a theoretical basis for the treatment and research of anastomotic leakage (13-15). Department of General Surgery of the Affiliated Cancer Hospital of Zhengzhou University summarized the relevant experiences of treating high anastomotic leakage after operation for AEG in our hospital and other hospital; classified anastomotic leakage according to the principles of timely detection, timely treatment, and minimal harm; and carried out relevant treatment according to different types. According to size and risk, anastomotic can be divided into types I, II, III, and IV, with the different types having different clinical characteristics. Type I (subclinical leakage) usually has no obvious clinical symptoms and can only be detected by upper gastrointestinal contrast swallow examination. If not detected in time, type I leakage is likely to develop into type II leakage, or even type III leakage, which requires surgical treatment. Typically, normal upper gastrointestinal contrast swallow examination in two consecutive 1-week intervals is considered to be the standard of successful treatment. For patients with type II (clinical leakage without surgical intervention) leakage, fever, abnormal drainage, and other clinical symptoms often occur. Upper digestive tract contrast swallow study can visualize the leakage. Improper management may also lead to the leakage requiring surgical intervention. Because intervention or color ultrasound puncture and drainage cannot be performed, type III leakage (clinical leakage requiring surgical intervention only) often requires surgical intervention. The main reasons for intervention are as follows: after leakage, the effusion enters the chest cavity, and the drainage tube cannot be placed in the chest cavity at the same time, or the drainage tube is placed. There also may be abdominal fluid after leakage, which cannot be drained smoothly through the drainage tube due to displacement of the drainage tube, removal of the drainage tube, or expansion of the drainage area. Additional surgery may be needed to clear the fluid and place a drain. Type IV leakage [clinical leakage requiring surgery and intensive care unit (ICU) intervention] includes patients with multiple organs, circulatory, or respiratory dysfunction due to leakage, with a very severe course of disease requiring drastic surgical intervention and postoperative ICU support.
Depending on the leakage type, different types of treatment can be implemented. For type I (subclinical leakage), the primary intervention is tube feeding with a nutrient tube, which generally does not require antibiotic anti-infective therapy. Gastrointestinal contrast swallow study and CT should be regularly reviewed to evaluate the healing of the leak. Generally, upper digestive tract contrast swallow examination is reviewed once a week, and it takes 3–4 weeks after surgery for the anastomotic leak to heal. Two consecutive reviews with contrast swallow study indicating a normal upper digestive tract are considered to be the standard criterion for successful treatment. However, attention should be paid to the CT examination to see if there is tension fluid around the leak, which may cause a false negative result. Type II (clinical leakage without surgical intervention) is mainly treated by unobstructed drainage under the guidance of intervention or color ultrasound, or use of endoscopic titanium clamp, endoscopic anastomosis clamp system (over-the-scope clips), biological glue blocking, or placement of biological scaffolds for treatment (16-18). Subsequent treatment consists of fasting, tube feeding, and antibiotics for anti-infection treatment as appropriate. Keep the drain flowing and clear (intermittent or continuous drainage can be performed). Because of the obvious clinical manifestations of leakage, a small amount of water can be taken through the mouth to keep the leakage there relatively clean, which is conducive to healing. However, dynamic monitoring should be combined with CT examination to avoid fluid accumulation around the leak. Type III (clinical leakage requiring only surgical intervention) anastomotic leakage often requires surgical intervention. The purpose of surgery is not to repair the leakage but mainly to remove the necrotic tissue around the anastomosis, clean up the effusion, and place the drainage tube to maintain unobstructed drainage around the anastomosis. Depending on the drug sensitivity of the drainage fluid, 1–2 antibiotics are often required for anti-infection treatment. In type IV leakage (clinical miss requiring surgery and ICU intervention), most patients of this type are complicated with abnormal respiratory and circulatory system function, which requires ICU stay to adjust the function of respiratory and circulatory system before treatment is administered to improve the degree of tolerance to surgery. If the patient’s vital signs are abnormal, they need to be admitted to the ICU for further treatment. After the patient’s vital signs were stable, surgery was selected for treatment. Depending on the drug sensitivity of the drainage fluid, multiple antibiotics or even antifungal drugs combined with anti-infection therapy are often required.
Although different surgeons performed the first operation, the severity of anastomotic leakage was also related to the surgical approach, preoperative complications, and surgical incision. The average time for diagnosis of types I and II leakages was shorter than types III and IV leakages, indicating that the symptoms of types III and IV leakages appeared later and that types I and II leakages may also be detected and treated at a late time; if left untreated, it may progress to types III or IV leakage. Types III and IV leakages tends to be larger, slow to heal, and involve a longer course of disease. The results also showed that the time to start oral eating and the first postoperative hospital stay for types III and IV leakages were significantly longer than those for types I and II leakages (P<0.05). Compared with that of types I and II leakages, the disease of types III and IV leakages was more serious and more often accompanied by peritonitis, bacteremia, cardiac insufficiency, pneumonia, and other complications; moreover, more than two doses of antibiotics or combination with antifungal drugs were more often required, with this difference being statistically significant. After statistical analysis of mortality within 90 days after surgery, it was found that the mortality rate of patients with types III and IV leakages was 14.8%, while there were no deaths in patients with types I and II leakages, and the difference in mortality rate was statistically significant.
Fistulas of types I and II can be successfully treated conservatively or with endoscopic methods, while types III and IV fistulas pose a threat to the patient’s life, resulting in severe complications and death. For this group of patients, the implementation of endoscopic techniques [such as endoscopic vacuum-assisted closure (EVAC), stenting, stent over sponge] remains the method of choice (19-21). Surgical intervention is recommended for fistulas diagnosed beyond 72 hours. In this treatment approach, closing the fistula in the anastomosis or performing re-anastomosis with or without lung decortication and removal of infected tissues from the pleural cavity, is recommended. This is aimed at controlling the inflammatory process in the posterior mediastinum.
Anastomotic leakage has always been an unavoidable clinical problem for general surgeons, especially superior anastomotic leakage after EGJ carcinoma surgery. The treatment process is complicated and long, and the clinical treatment outcome is extremely difficult to control. In this paper, superior anastomotic leakage is classified, and various treatment schemes are recommended with the aim of providing a reference for general surgeons in the diagnosis and treatment of postoperative anastomotic leakage for gastric cancer. Due to the small number of cases and the single-center design, the results of this study may involve selection bias and certain limitations.
Conclusions
With the continuous development of biomaterials, intervention, endoscopy, and other medical technologies, general surgeons need to constantly learn and master new technologies, and continue to think, discuss, and pool their wisdom to develop more appropriate treatment programs for patients with superior anastomotic leakage after EGJ carcinoma surgery.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-968/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-968/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-968/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-968/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of the Affiliated Cancer Hospital of Zhengzhou University (No. 2021-132-006) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Quante M, Abrams JA, Lee Y, et al. Barrett esophagus: what a mouse model can teach us about human disease. Cell Cycle 2012;11:4328-38. [Crossref] [PubMed]
- Watanabe M, Ito H, Hosono S, et al. Declining trends in prevalence of Helicobacter pylori infection by birth-year in a Japanese population. Cancer Sci 2015;106:1738-43. [Crossref] [PubMed]
- Yan CY, Chen LQ. Retrospect of 2019: focus on the surgical treatment for adenocarcinoma of esophagogastric junction. Zhonghua Wei Chang Wai Ke Za Zhi 2020;23:20-5. [PubMed]
- Kammili A, Ramirez-GarciaLuna JL, Mueller CL, et al. Personalized surgical management of esophagogastric junction cancers: retrospective cohort study at a Canadian institution. Ann Esophagus 2021;4:24. [Crossref]
- Ubels S, Verstegen MHP, Rosman C, et al. Anastomotic leakage after esophagectomy for esophageal cancer: risk factors and operative treatment. Ann Esophagus 2021;4:8. [Crossref]
- Turrentine FE, Denlinger CE, Simpson VB, et al. Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg 2015;220:195-206. [Crossref] [PubMed]
- Schuchert MJ, Abbas G, Nason KS, et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery 2010;148:831-8; discussion 838-40. [Crossref] [PubMed]
- Ma Z, Chen C, Shang X, et al. Comparison of lymph node metastasis pattern from esophagogastric junction adenocarcinoma versus very low thoracic esophageal squamous cancer: a propensity-matched analysis. J Thorac Dis 2023;15:442-51. [Crossref] [PubMed]
- Nagasako Y, Satoh S, Isogaki J, et al. Impact of anastomotic complications on outcome after laparoscopic gastrectomy for early gastric cancer. Br J Surg 2012;99:849-54. [Crossref] [PubMed]
- Tsou CC, Lo SS, Fang WL, et al. Risk factors and management of anastomotic leakage after radical gastrectomy for gastric cancer. Hepatogastroenterology 2011;58:218-23. [PubMed]
- Li QG, Li P, Tang D, et al. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J Gastroenterol 2013;19:4060-5. [Crossref] [PubMed]
- Yoo HM, Lee HH, Shim JH, et al. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J Surg Oncol 2011;104:734-40. [Crossref] [PubMed]
- Hu JK, Yang K. Prevention and treatment of anastomosis-related complications after gastrectomy for gastric cancer. Chinese Journal of Practical Surgery 2017;37:362-6.
- Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001;88:1157-68. [Crossref] [PubMed]
- Lerut T, Coosemans W, Decker G, et al. Anastomotic complications after esophagectomy. Dig Surg 2002;19:92-8. [Crossref] [PubMed]
- Rodella L, Laterza E, De Manzoni G, et al. Endoscopic clipping of anastomotic leakages in esophagogastric surgery. Endoscopy 1998;30:453-6. [Crossref] [PubMed]
- Lippert E, Klebl FH, Schweller F, et al. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int J Colorectal Dis 2011;26:303-11. [Crossref] [PubMed]
- Haito-Chavez Y, Law JK, Kratt T, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc 2014;80:610-22. [Crossref] [PubMed]
- Virgilio E, Ceci D, Cavallini M. Surgical Endoscopic Vacuum-assisted Closure Therapy (EVAC) in Treating Anastomotic Leakages After Major Resective Surgery of Esophageal and Gastric Cancer. Anticancer Res 2018;38:5581-7. [Crossref] [PubMed]
- Kim YI, Kim CG, Lee JY, et al. Effectiveness of a Novel Covered Stent without External Thread Fixation for Anastomotic Leakage after Total or Proximal Gastrectomy for Gastric Cancer. Cancers (Basel) 2021;13:3720. [Crossref] [PubMed]
- Gubler C, Schneider PM, Bauerfeind P. Complex anastomotic leaks following esophageal resections: the new stent over sponge (SOS) approach. Dis Esophagus 2013;26:598-602. [Crossref] [PubMed]


