
Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as biomarkers to prognosticate survival in advanced gastric cancer patients in the era of immunotherapy: a systematic review and meta-analysis
Highlight box
Key findings
• There is potential for the use of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic biomarkers for advanced gastric cancer (GC) patients receiving immunotherapy, with elevated levels associated with poorer survival outcomes.
What is known and what is new?
• Immunotherapy is increasingly used for advanced GC, but predicting treatment outcomes remains challenging.
• This study presents evidence supporting the use of novel inflammatory markers, NLR and PLR, as potential prognostic indicators for advanced GC patients undergoing immunotherapy, shedding light on their predictive value.
What is the implication, and what should change now?
• Incorporating NLR and PLR assessments into routine clinical practice for advanced GC patients receiving immunotherapy could enhance prognostic accuracy and guide treatment decisions.
Introduction
Gastric cancer (GC) remains the fourth most commonly diagnosed malignancy and the fourth leading cause of cancer-related death, with approximately 990 thousand diagnoses and 800 thousand deaths per year according to worldwide estimates (1,2). Approximately two-thirds of newly diagnosed cases are locally advanced, metastatic, or unresectable, and the 5-year overall survival (OS) for these patients is short (3).
Chemotherapy is the long-standing standard first-line treatment for advanced GC; however, treatment options for these patients have evolved in recent years. The CheckMate 649 trial found that nivolumab added to chemotherapy was associated with increased OS in human epidermal growth factor receptor 2 (HER2)-negative disease compared to chemotherapy alone (4). Additionally, the KEYNOTE-811 phase 3 study demonstrated that pembrolizumab added to chemotherapy and trastuzumab was associated with improvement in the objective response rate in the first-line setting for HER2-positive advanced GC (5). As a result, nivolumab and pembrolizumab were listed as first-line treatment options for HER2-negative and HER2-positive advanced GC, respectively, in the 2022 National Comprehensive Cancer Network (NCCN) guidelines (6).
Treatment options for advanced GC in later lines diverge according to guidelines. The ATTRACTION-2 trial demonstrated that nivolumab was associated with improved OS in advanced GC patients who were chemo-refractory to first- and second-line treatments (7). This prompted the incorporation of nivolumab as a third-line treatment option in the Asian setting for patients who underwent no previous treatment with immune checkpoint inhibitors (ICIs) (8-10). Currently, both NCCN and European Society for Medical Oncology (ESMO) guidelines for advanced GC do not recommend ICI use as third-line treatment (6,11).
Despite the increasing use of immunotherapy in advanced GC, relevant questions remain concerning the limited efficacy of drugs in a subset of patients, drug-related toxicity, and cost-associated considerations. While the combined predictive score has been widely adopted to define eligibility for ICI therapy, some studies suggest that the treatment efficacy of immunotherapy agents may be irrespective of the patient’s programmed cell death protein ligand 1 (PD-L1) status (12,13). Moreover, some data reported poor survival outcomes in subsets of advanced GC patients who were eligible for ICI treatment (14). Taken together, these factors prompt a thorough search for biomarkers that can predict ICI efficacy.
Epstein-Barr virus status, tumor mutation burden, and microsatellite instability have been described as biomarkers of ICI effectiveness (15-17). Nevertheless, the diversity of epidemiologic prevalence, substantial associated costs, and invasiveness of certain tests hinder the universal implementation of these biomarkers for GC patients (18-21). New, easily available, and cost-effective biomarkers are needed to predict outcomes in immune checkpoint blockade in advanced GC.
In recent years, novel inflammatory markers derived from complete blood count measurements have gained considerable attention for their potential as indicators of disease severity and prognosis. The platelet-to-lymphocyte ratio (PLR) is an emerging inflammatory marker derived from complete blood count measures. It has been studied as a prognostic, diagnostic, and disease severity marker in diverse conditions, such as chronic obstructive pulmonary disease (COPD), coronavirus disease (COVID), and ankylosing spondylitis (22-24). In oncology, the hypothesis of higher PLR values correlating with worse survival outcomes was investigated in several malignancies with conflicting results (25-28).
Similarly, the neutrophil-to-lymphocyte ratio (NLR) is another novel inflammatory measure that is also obtainable from complete blood count measurements. Several studies have demonstrated its use as a reliable predictor of severity for diverse conditions, including sepsis, COVID 2019 (COVID-19), and acute pancreatitis (24,29,30). In oncology, the NLR has been studied in numerous malignancies as a diagnostic and prognostic tool (31-33). In recent years, several studies have explored the potential of NLR as a prognostic biomarker for advanced GC treated with immunotherapy, and the results vary. Two meta-analyses have been published on the matter, with partly divergent findings. Since their inception, new studies have emerged. Our primary aim in this study is to conduct a systematic review and meta-analysis to comprehensively assess the predictive roles of novel inflammatory markers—PLR and NLR—as indicators of OS and progression-free survival (PFS) in patients with advanced GC and gastroesophageal junction cancer (GEJC) undergoing immunotherapy. Despite evolving treatment options and the increasing utilization of immunotherapy in these patients, our study intends to address the persistent need for reliable biomarkers to predict treatment efficacy and outcomes in this specific therapeutic landscape. For NLR, this will be an updated study, while for PLR, this study will mark the inaugural meta-analysis in this population. We present this article in accordance with the PRISMA reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-808/rc).
Methods
We conducted a systematic search of eligible articles published up to 3 April 2023 in the following databases: Cochrane Library, Embase, and PubMed. The designed search strategy consisted of words, characters, and Boolean operators as follows: (“plr” OR “nlr” OR “platelet” OR “neutrophil”) AND (“gastric” OR “gastrointestinal”) AND (“immunotherapy” OR “PD” OR “checkpoint” OR “nivolumab” OR “pembrolizumab” OR “ipilimumab OR “atezolizumab” OR “avelumab” OR “durvalumab”). Additional filters were applied to Embase to narrow down the results to gastric neoplasms. A list of articles was generated from each database and imported into Rayyan software, where duplicates were removed manually (34). The “Blind Mode” was activated, and two independent investigators screened studies for inclusion. Conflicting decisions were resolved by a third reviewer.
PICOS strategy used for this study is as follows: (I) population: patients with advanced GC/GEJC treated with immunotherapy; (II) intervention: patients with biomarker value equal or higher to cut-off; (III) control: patients with biomarker value less than cut-off; (IV) outcome: OS and PFS; and (V) study design: prospective and retrospective studies comparing low biomarker group vs. high biomarker. This meta-analysis was registered in the PROSPERO network with the following ID: CRD42023460928.
Selection criteria
Inclusion criteria were as follows: (I) the population encompassed advanced GC/GEJC patients who received immunotherapy as their treatment regimen, irrespective of treatment line; (II) examined the prognostic significance of baseline NLR or PLR in relation to OS or PFS; (III) presented hazard ratio (HR) along with its corresponding 95% confidence interval (CI); (IV) study from any country, written in English language; and (V) full text articles or conference abstracts. Exclusion criteria were as follows: (I) studies not providing data on the targeted population; (II) comprehensive studies encompassing other cancer types and not providing distinct datasets for advanced GC/GEJC subgroups; (III) studies featuring intersecting populations and data regarding the same biomarker were subject to analysis, with the article containing the smaller patient cohort being excluded; and (IV) expert opinions, reviews, nonhuman studies or case reports.
Data extraction
Two independent reviewers carefully extracted data as follows: first author surname, year of publication, country of origin, NLR cutoff value, PLR cutoff value, methods of determining cutoff value, number of patients enrolled, center design, median follow-up, median age of patients and ICI type. Subsequently, the data were imported into Microsoft Excel (2019 version). Inconsistencies were subsequently resolved by a third reviewer. The evaluation of quality was conducted employing the Newcastle-Ottawa scale (NOS), wherein specific ratings were assigned to each study included. Studies attaining scores of 7 or greater were categorized as exhibiting high quality.
Statistical analysis
HRs and their corresponding 95% CIs were pooled employing the generic inverse variance and random-effects model. Heterogeneity was assessed using the Higgins I2 model, wherein values of I2 exceeding 50% represented substantial heterogeneity. Subgroup analyses were also conducted in this manner. Publication bias was assessed using visual inspection of funnel plots and their triangular region. All statistical tests were two-sided and were considered significant if P<0.05. All analyses were undertaken utilizing Review Manager version 5.4.
Results
Selection process
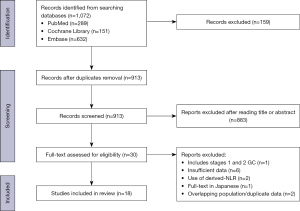
A total of 1,072 articles were obtained from the three databases. Following the elimination of duplicate entries, 913 articles underwent initial screening based on their titles and abstracts, of which 883 were found to be unrelated to the subject matter under review. Subsequently, a detailed examination of the full texts of the remaining 30 studies was carried out, leading to the exclusion of 12 studies in accordance with our predefined exclusion criteria. Ultimately, this systematic review included 18 studies (12,35-51). Our selection process is summarized in Figure 1.
Study characteristics
Predominantly conducted within Asia, the studies were distributed as follows: 9 studies in China, 7 in Japan, and 1 in Korea. The sole non-Asian study was conducted by Formica et al. (35) This collection of studies spans the years between 2018 and 2023. Sixteen studies investigated the prognostic value of NLR, while 8 investigated PLR. Among the articles regarding NLR, 14 included NLR prognostic values for OS and 11 for PFS. Among the studies investigating PLR, 7 included reporting of the prognostic values for OS and 6 for PFS. A diverse range of cutoff values was adopted by the included articles, varying from 2.5 to 5.0 for NLR and from 139.41 to 267.00 for PLR. Detailed methods for establishing cutoffs, as well as general information, are outlined in Table 1. Notably, Qu et al. (50) performed distinct analyses for patients receiving immunotherapy as a first-line treatment and those treated in later lines. This prompted us to treat that investigation as two separate studies, each subjected to its own independent analysis. Therefore, we named the two groups “Qu (I)” for the first line and “Qu (II)” for the second and later lines. Gou et al. (44) conducted two studies with overlapping populations, one of which included reporting of results for the NLR role as a prognostic tool, while the other did the same for the PLR (42,44). We decided to include both studies since each one would pertain to separate analyses, one for NLR [named “Gou (I)”] and the other for PLR [named “Gou (II)”].
Table 1
| Study | Year | Study period | Country | Type of study | Median follow-up | No. of patients | Biomarker | Cutoff value | Cutoff method | Survival analysis | Outcomes | ICI | Median age (years) | Center design |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tanaka et al. | 2022 | Oct 2017–Oct 2019 | Japan | Prospective | 1 year | 70 | NLR | 5.00 (NLR) | Unclear | Multivariate | OS | Nivolumab | 69 | Multicentric |
| Ruan et al. | 2021 | Dec 2016–Sep 2017 | China | Prospective | 4.5 months | 58 | NLR, PLR | 2.70 (NLR), 267.00 (PLR) | ROC curve | Multivariate | OS, PFS | Toripalimab | 60 | Multicentric |
| Kim et al. | 2022 | Nov 2014–Feb 2016 | Korea | Prospective | 28.3 months | 45 | NLR | 2.90 (NLR) | Median | Univariate (OS), multivariate (PFS) | OS, PFS | Nivolumab | 60 | Single-center |
| Xiang et al. | 2022 | Dec 2020–Apr 2021 | China | Retrospective | 14.2 months | 103 | NLR | 2.38 (NLR) | ROC curve | Multivariate | PFS | Camrelizumab | 62 | Single-center |
| Wan et al. | 2022 | Dec 2017–Dec 2020 | China | Retrospective | 27.3 months | 45 | NLR, PLR | 3.85 (NLR), 214.08 (PLR) | ROC curve | Multivariate | OS, PFS | Camrelizumab, sintilimab, tislelizumab, toripalimab, envafolimab, CS1001, HX008 | 64 | Single-center |
| Gou (I) et al. | 2021 | Jan 2016–Jan 2020 | China | Retrospective | Unclear | 137 | NLR | 3.23 (NLR) | ROC curve | Multivariate | OS, PFS | Nivolumab, pembrolizumab, sintilimab, toripalimab | 59 | Single-center |
| Gou (II) et al. | 2022 | Oct 2016–Aug 2021 | China | Retrospective | Unclear | 237 | PLR | 139.41 (PLR) | ROC curve | Multivariate | OS, PFS | Nivolumab, pembrolizumab, sintilimab, toripalimab | 59 | Single-center |
| Yamada et al. | 2020 | Dec 2014–Feb 2019 | Japan | Retrospective | 175 days | 89 | NLR | 2.50 (NLR) | ROC curve | Multivariate | OS, PFS | Nivolumab | Unclear | Single-center |
| Ota et al. | 2020 | Dec 2014–Sep 2018 | Japan | Retrospective | 4.9 months | 98 | NLR | 3.00 (NLR) | ROC curve | Multivariate (OS), univariate (PFS) | OS, PFS | Nivolumab | 66 | Single-center |
| Ogata et al. | 2018 | Jun 2017–Dec 2017 | Japan | Retrospective | 171 days | 26 | NLR | 5.00 (NLR) | Literature | Univariate | OS, PFS | Nivolumab | 64 | Multicentric |
| Hayano et al. | 2023 | Oct 2018–Dec 2021 | Japan | Retrospective | Unclear | 70 | NLR, PLR | 2.42 (NLR), 152.50 (PLR) | Unclear | Univariate | PFS | Nivolumab, pembrolizumab | 71 | Single-center |
| Li et al. | 2023 | May 2015–May 2021 | China | Retrospective | 9.4 months | 54 | NLR, PLR | Unclear | Median | Multivariate | OS | Nivolumab, pembrolizumab | 58 | Single-center |
| Sakai et al. | 2022 | Sep 2017–Mar 2020 | Japan | Retrospective | 150 days | 117 | NLR | 2.54 (NLR) | Median | Multivariate | OS | Nivolumab | 71 | Multicentric |
| Qu (I) et al. | 2022 | Jul 2019–Mar 2020 | China | Retrospective | 17.5 months | 53 | NLR, PLR | 3.11 (NLR), 243.33 (PLR) | ROC curve | Multivariate | OS, PFS | Camrelizumab, sintilimab, toripalimab, pembrolizumab, nivolumab | Unclear | Single-center |
| Qu (II) et al. | 2022 | Jul 2019–Mar 2020 | China | Retrospective | 15.9 months | 53 | NLR, PLR | 3.11 (NLR), 243.33 (PLR) | ROC curve | Multivariate | OS, PFS | Camrelizumab, sintilimab, toripalimab, pembrolizumab, nivolumab | Unclear | Single-center |
| Suzuki et al. | 2021 | Oct 2017–Feb 2019 | Japan | Retrospective | 4.8 months | 72 | NLR | 5.00 (NLR) | Unclear | Multivariate | OS | Nivolumab | 70 | Multicentric |
| Namikawa et al. | 2020 | Oct 2017–Dec 2019 | Japan | Retrospective | 32 months | 29 | NLR | 2.50 (NLR) | Median | Univariate | OS, PFS | Nivolumab | 71 | Single-center |
| Formica et al. | 2020 | Jun 2014–Dec 2018 | England | Retrospective | 27 months | 57 | NLR, PLR | Continuous (NLR), 200.00 (PLR) | Unclear | Multivariate | OS | Nivolumab, pembrolizumab, avelumab | 63 | Multicentric |
| Chen et al. | 2022 | Aug 2015–Apr 2019 | China | Retrospective | 23.8 months | 139 | PLR | 173.70 (PLR) | ROC curve | Multivariate | OS, PFS | Undisclosed | 60 | Single-center |
ICI, immune checkpoint inhibitor; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PLR, platelet-to-lymphocyte ratio; ROC, receiver operating characteristic; PFS, progression-free survival.
Analysis
In this meta-analysis, we investigated the predictive significance of differentiating between low and high biomarker values (NLR and PLR) concerning OS and PFS. Our OS analysis involved pooling HRs and their corresponding 95% CIs by comparing low biomarker values with high biomarker values across the included studies. Whenever feasible, we prioritized multivariate HR and 95% CI data but also incorporated univariate data if it was the only available information. A similar process was employed in the analysis of PFS.
Analysis for OS
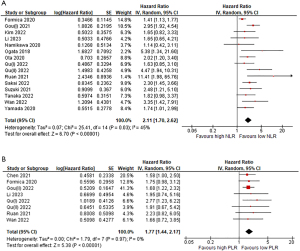
In relation to NLR, 10 out of the 15 studies included reporting of a significant HR for OS, indicating a statistically significant association between a high NLR and worse OS prognosis. Our pooled analysis resulted in an HR of 2.11 with 95% CI (1.70–2.62) and a heterogeneity of 45%.
Regarding PLR, only 3 out of the 8 studies presented a significant HR and 95% CI, indicating a significant association between high PLR and unfavorable OS prognosis. Our pooled analysis yielded an HR of 1.77 with 95% CI (1.44–2.17) and a heterogeneity of 0%. The results are shown in Figure 2.
Analysis of PFS
In relation to NLR, only 5 out of the 12 studies included reporting of a significant HR for PFS. Although not significant, the results of Namikawa et al. (47) tended to suggest that high NLR values were associated with better PFS outcomes in contrast to other studies. Our pooled analysis resulted in an HR of 1.76 with 95% CI (1.43–2.17) and a heterogeneity of 25%.
Regarding PLR, only 3 out of the 8 studies presented a significant HR and 95% CI for the PFS analysis, indicating a significant association between high PLR and unfavorable PFS prognosis. Our pooled analysis yielded an HR of 1.61 with 95% CI (1.33–1.96) and no heterogeneity (0%). The results are shown in Figure 3.

Subgroup analysis
The wide range of cutoff values across studies, different countries of origin, different study designs, and variation in follow-up periods rendered a subgroup analysis suitable for our systematic review. Both OS and PFS data underwent subgroup analysis. The data were categorized based on NLR cutoff values (≥3 or <3) or PLR cutoff values (>200 or ≤200), the country of origin, follow-up period (<12 or ≥12 months), sample size (>80 or ≤80), survival analysis approach, study design, and the number of centers involved (1 or >1). The outcomes of NLR subgroup analyses are presented in Tables 2,3. The outcomes of PLR subgroup analyses are presented in Tables 4,5.
Table 2
| Subgroups | Number of studies | Effects model | HR (95% CI) | P | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | P | |||||
| Cutoff | ||||||
| ≥3 | 8 | Random | 2.48 (1.96–3.15) | <0.001 | 6 | 0.39 |
| <3 | 5 | Random | 1.96 (1.32–2.89) | <0.001 | 32 | 0.21 |
| Continuous | 1 | 1.41 (1.13–1.77) | 0.002 | |||
| Undisclosed | 1 | 1.65 (0.65–4.21) | 0.29 | |||
| Country | ||||||
| Japan | 7 | Random | 2.04 (1.61–2.58) | <0.001 | 0 | 0.65 |
| China | 6 | Random | 2.74 (1.88–4.14) | <0.001 | 36 | 0.17 |
| Korea | 1 | 1.67 (0.82–3.33) | 0.16 | |||
| England | 1 | 1.41 (1.13–1.77) | 0.002 | |||
| Survival analysis | ||||||
| Multivariate | 11 | Random | 2.23 (1.73–2.88) | <0.001 | 54 | 0.02 |
| Univariate | 4 | Random | 1.72 (1.11–2.66) | 0.01 | 9 | 0.35 |
| Sample size | ||||||
| ≥80 | 4 | Random | 2.29 (1.80–2.92) | <0.001 | 0 | 0.46 |
| <80 | 11 | Random | 2.08 (1.54–2.82) | <0.001 | 48 | 0.04 |
| Center | ||||||
| Multicenter | 6 | Random | 2.19 (1.46–3.27) | <0.001 | 60 | 0.03 |
| Single center | 9 | Random | 2.15 (1.79–2.65) | <0.001 | 19 | 0.28 |
| Study design | ||||||
| Retrospective | 12 | Random | 2.12 (1.67–2.69) | <0.001 | 48 | 0.03 |
| Prospective | 3 | Random | 2.25 (1.09–4.65) | 0.03 | 52 | 0.12 |
| Follow-up | ||||||
| <12 months | 6 | Random | 2.23 (1.56–3.19) | <0.001 | 20 | 0.28 |
| ≥12 months | 8 | Random | 1.90 (1.44–2.50) | <0.001 | 44 | 0.09 |
| Unclear | 1 | 2.94 (1.92–4.54) | <0.001 | |||
NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Table 3
| Subgroups | Number of studies | Effects model | HR (95% CI) | P | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | P | |||||
| Cutoff | ||||||
| ≥3 | 6 | Random | 1.80 (1.37–1.38) | <0.001 | 0 | 0.64 |
| <3 | 6 | Random | 1.64 (1.12–2.39) | 0.01 | 48 | 0.08 |
| Country | ||||||
| Japan | 5 | Random | 1.33 (1.01–1.77) | 0.04 | 10 | 0.35 |
| China | 6 | Random | 2.21 (1.71–2.84) | <0.001 | 0 | 0.80 |
| Korea | 1 | 2.17 (1.05–4.54) | 0.037 | |||
| Survival analysis | ||||||
| Multivariate | 7 | Random | 1.96 (1.47–2.62) | <0.001 | 35 | 0.16 |
| Univariate | 5 | Random | 1.49 (1.12–1.99) | 0.006 | 0 | 0.50 |
| Sample size | ||||||
| ≥80 | 4 | Random | 1.74 (1.11–2.72) | 0.02 | 67 | 0.03 |
| <80 | 8 | Random | 1.82 (1.41–2.35) | <0.001 | 0 | 0.62 |
| Center | ||||||
| Multicenter | 2 | Random | 2.21 (1.33–3.69) | 0.002 | 0 | 0.82 |
| Single center | 10 | Random | 1.70 (1.34–2.17) | <0.001 | 34 | 0.13 |
| Study design | ||||||
| Retrospective | 10 | Random | 1.70 (1.33–2.17) | <0.001 | 34 | 0.13 |
| Prospective | 2 | Random | 2.16 (1.35–3.43) | 0.001 | 0 | 0.96 |
| Follow-up | ||||||
| <12 months | 4 | Random | 1.51 (1.07–2.12) | 0.02 | 30 | 0.23 |
| ≥12 months | 6 | Random | 1.89 (1.31–2.75) | <0.001 | 23 | 0.26 |
| Unclear | 2 | Random | 2.05 (1.49–2.83) | <0.001 | 2 | 0.31 |
NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval.
Table 4
| Subgroups | Number of studies | Effects model | HR (95% CI) | P | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | P | |||||
| Cutoff | ||||||
| >200 | 4 | Random | 2.13 (1.35–3.35) | 0.001 | 0 | 0.85 |
| ≤200 | 3 | Random | 1.67 (1.31–2.12) | <0.0001 | 0 | 0.96 |
| Undisclosed | 1 | 1.96 (0.74–5.16) | ||||
| Country | ||||||
| China | 7 | Random | 1.77 (1.42–2.21) | <0.001 | 0 | 0.94 |
| England | 1 | 1.75 (0.98–3.12) | 0.06 | |||
| Survival analysis | ||||||
| Multivariate | 6 | Random | 1.85 (1.47–2.33) | <0.001 | 0 | 0.58 |
| Univariate | 2 | Random | 1.72 (1.07–2.77) | 0.03 | 0 | 0.92 |
| Sample size | ||||||
| ≥80 | 2 | Random | 1.65 (1.27–2.15) | <0.001 | 0 | 0.83 |
| <80 | 6 | Random | 1.97 (1.41–2.76) | <0.001 | 0 | 0.96 |
| Center | ||||||
| Multicenter | 2 | Random | 1.86 (1.13–3.07) | 0.02 | 0 | 0.68 |
| Single center | 6 | Random | 1.75 (1.39–2.19) | <0.001 | 0 | 0.90 |
| Study design | ||||||
| Retrospective | 7 | Random | 1.75 (1.41–2.16) | <0.001 | 0 | 0.95 |
| Prospective | 1 | 2.22 (0.82–6.05) | 0.12 | |||
| Follow-up | ||||||
| <12 months | 2 | Random | 2.08 (1.04–4.18) | 0.04 | 0 | 0.85 |
| ≥12 months | 5 | Random | 1.89 (1.42–2.53) | <0.001 | 0 | 0.55 |
| Unclear | 1 | 1.69 (1.22–2.32) | 0.001 | |||
PLR, platelet-to-lymphocyte ratio; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Table 5
| Subgroups | Number of studies | Effects model | HR (95% CI) | P | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | P | |||||
| Cutoff | ||||||
| >200 | 4 | Random | 1.90 (1.29–2.80) | 0.001 | 0 | 0.50 |
| ≤200 | 3 | Random | 1.53 (1.22–1.91) | <0.0001 | 0 | 0.45 |
| Country | ||||||
| China | 6 | Random | 1.65 (1.34–2.03) | <0.001 | 0 | 0.47 |
| Japan | 1 | 1.39 (0.81–2.38) | 0.2 | |||
| Survival analysis | ||||||
| Multivariate | 4 | Random | 1.68 (1.29–2.18) | <0.001 | 14 | 0.32 |
| Univariate | 3 | Random | 1.47 (1.01–2.14) | 0.04 | 0 | 0.58 |
| Sample size | ||||||
| ≥80 | 2 | Random | 1.53 (1.13–2.08) | 0.007 | 31 | 0.23 |
| <80 | 5 | Random | 1.71 (1.25–2.34) | <0.001 | 0 | 0.52 |
| Center | ||||||
| Multicenter | 1 | 1.16 (0.54–2.50) | 0.71 | |||
| Single center | 6 | Random | 1.65 (1.35–2.01) | <0.001 | 0 | 0.53 |
| Study design | ||||||
| Retrospective | 6 | Random | 1.65 (1.35–2.01) | <0.001 | 0 | 0.53 |
| Prospective | 1 | 1.16 (0.54–2.50) | 0.71 | |||
| Follow-up | ||||||
| <12 months | 1 | 1.16 (0.54–2.50) | 0.709 | |||
| ≥12 months | 4 | Random | 1.73 (1.21–2.48) | 0.003 | 18 | 0.30 |
| Unclear | 2 | Random | 1.64 (1.26–2.13) | <0.001 | 0 | 0.49 |
PLR, platelet-to-lymphocyte ratio; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval.
Risk of bias
We evaluated potential bias employing the Newcastle-Ottawa approach, scrutinizing three key domains: selection (0–4 points), comparability (0–2 points), and outcome (0–3 points). Each included article was appraised by two independent reviewers. Any disparities in the assigned scores were addressed through the involvement of a third reviewer to ensure consensus. The outcomes are presented in Table 6. In the selection domain, all studies achieved the highest score. This was because in each study, both arms were sourced from an identical cohort, and the clinical data were acquired from secure medical records. The majority of studies obtained the highest score within the Comparability domain, as they applied multivariate analysis for the evaluated outcomes. Some studies lost points in the outcome domain due to short or undisclosed follow-up periods. In summary, all the studies were deemed to be of high quality, with the lowest assigned score being 7.
Table 6
| Authors | Selection | Comparability | Outcome | Total score |
|---|---|---|---|---|
| Tanaka et al. | **** | ** | *** | 9 |
| Ruan et al. | **** | ** | ** | 8 |
| Kim et al. | **** | * | *** | 8 |
| Xiang et al. | **** | ** | ** | 8 |
| Wan et al. | **** | ** | *** | 9 |
| Gou (I) et al. | **** | ** | ** | 8 |
| Gou (II) et al. | **** | ** | ** | 8 |
| Yamada et al. | **** | ** | ** | 8 |
| Ota et al. | **** | ** | ** | 8 |
| Ogata et al. | **** | * | ** | 7 |
| Hayano et al. | **** | * | ** | 7 |
| Li et al. | **** | ** | ** | 8 |
| Sakai et al. | **** | ** | *** | 9 |
| Qu et al. | **** | ** | *** | 9 |
| Suzuki et al. | **** | ** | ** | 8 |
| Namikawa et al. | **** | * | ** | 8 |
| Formica et al. | **** | ** | *** | 9 |
| Chen et al. | **** | ** | *** | 9 |
Each asterisk (*) represents 1 point in NOS score. NOS, Newcastle-Ottawa scale.
Publication bias
Figures 4,5 display the NLR and PLR funnel plots for OS and PFS, respectively. Considering NLR funnel plots, visual inspection revealed a high possibility of publication bias for OS analysis, with the plot showing asymmetry and the Formica et al. (35) study falling outside the funnel. A low possibility of publication bias for PFS analysis can be concluded based on the minor asymmetry depicted in the funnel plot, with the study falling within the triangular region.

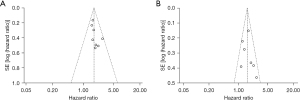
In relation to PLR funnel plots, visual inspection revealed a low possibility of publication bias for both OS and PFS. In both analyses, minor asymmetry was found, and all included studies fell within the triangular region.
Discussion
This meta-analysis aimed to investigate PLR and NLR roles in predicting the prognosis of advanced GC patients treated with immunotherapy. Conflicting data across studies prompted a thorough investigation into this matter. Our investigation evaluated the prognostic value of baseline NLR in 16 studies comprising 1,176 patients. Our study found correlations between high NLR values and shorter OS and between high NLR values and shorter PFS, with HRs and P values indicating significant correlations. Our meta-analysis assessed the prognostic value of the PLR in advanced GC/GEJC patients treated with immunotherapy across eight studies encompassing 766 patients. To the best of our knowledge, this is the first systematic review to investigate the role of PLR in this population. The pooled results unveiled a significant association between high PLR and poor OS and between high PLR and poor PFS. Remarkably, heterogeneity was null for both PLR analyses, highlighting the low risk of bias related to our findings. Our findings are in line with many of the included studies conclusions regarding association between high biomarker value and worsened survival.
Our study comes in light of two previous meta-analyses that investigated the prognostic role of NLR in our target population. In their meta-analysis of nine studies, Zhang et al. (52) found a significantly poorer OS for patients with high NLR but failed to find a significant relationship between high NLR and poor PFS. Within a short period of time after this study was published, Li et al. (53) published another meta-analysis investigating the same group of patients in 10 studies. In this systematic review, significant associations were found between poor OS prognosis and high NLR values and between poor PFS prognosis and high NLR values. The discrepant results regarding PFS prompted us to consider the need for further investigation. Moreover, additional studies have been published since their search deadlines. Our results are consistent with those of Li et al. (53), with shorter P values and stricter 95% CIs for PFS analysis.
For the sake of clarity, it is worth briefly discussing the possible mechanisms that may explain how inflammation and the novel inflammatory markers PLR and NLR influence cancerization and disease progression.
Inflammation is a pivotal process that contributes to the establishment of the cancer microenvironment and persistent tumor cell proliferation (54). Inflammatory pathways have been recognized as factors that can influence responses to drug treatments in cancer (55). Therefore, it is imperative to study inflammatory biomarkers in oncology, as these could potentially play a crucial role in diagnosing and prognosticating malignancies.
The NLR is an emerging biomarker, and the reasons underlying its prognostic utility as an inflammatory marker are incompletely understood. Emerging evidence links tumor-infiltrating neutrophils as key cells in promoting an immunosuppressive state by upregulating PD-L1 on cancer cells (56,57). Wang et al. (58) demonstrated a significant positive correlation between the expression of CD54, a neutrophil activating protein, and PD-L1 neutrophils from GC patients, highlighting the possible interplay between neutrophils and cancer-induced immunosuppression. Although some differences in gene and protein expression have been described between intratumoral and peripheral neutrophils, the scholars also reported an association between higher levels of peripheral neutrophils in comparison to healthy subjects, suggesting a link between the two cell populations (58). Indeed, in a 2021 study, Ruan et al. (51) identified a strong association between higher levels of enriched intratumoral neutrophils in GC patients with a high baseline NLR compared to those with a low NLR. Moreover, higher expression of biomarkers related to neutrophil recruitment and plasticity was reported in patients with a high NLR (51). CD4 and CD8 lymphocytes have been described in tumor cell destruction through immunosurveillance, a process of identifying and eliminating immunogenic cancer cell clones (59). A higher NLR translates in a degree of immunosurveillance loss and there is a tendency to interpret this biomarker as a surrogate for such a decline. The possible implications are not only the use of NLR as a baseline marker to assess prognostic, but as a dynamic tool to monitor immune status during treatment.
PLR is another novel inflammatory marker, and its role as a prognostic tool for several types of cancer remains controversial. Notably, there are several mechanisms explaining the interaction between tumor cells and platelets during metastization. One of the primary mechanisms involves the activation of platelets by tumor cells through the release of substances such as ADP, TXA2, and chemokines (60). In addition to these interactions, platelets are implicated in shielding circulating tumor cells from recognition by the immune system and in facilitating invasion of healthy tissues (61). In contrast to these factors, CD8+ T lymphocytes are recognized as the main players in the immune response against malignancies, developing complex crosstalk with surrounding immune cells in the tumor microenvironment and influencing inflammatory responses (62). The reasons surrounding the role of the PLR in tumor prognosis are incompletely understood, but these factors may help elucidate its role since both components, platelets, and lymphocytes, are implicated in cancer dynamics.
It is challenging to draw final conclusions for both NLR and PLR solely based on our findings. Ideally, the results from a systematic review in addition to individual patient data could provide a better understanding of the real prognostic role of NLR and PLR. Here, we seek to assess whether their significance is relevant in the era of immunotherapy. A number of studies have explored the role of NLR in advanced GC patients treated with chemotherapy, the traditional regimen for advanced disease. Many of these have resulted in findings that suggest a possible role in prognosticating survival outcomes (63-65). When considered collectively, our findings indicate the continued utility of NLR continuity in the context of immunotherapy. Data for the predictive role of PLR in chemotherapy are scarce and conflicting, with some studies indicating a potential use and others presenting inconclusive findings (66,67). Similarly, our meta-analysis assessment of PLR consisted of studies that had small sample sizes and were retrospective in nature.
One concern that arose in the aftermath of our statistical data completion was the limited follow-up of some of the included studies and the effect this could have on our conclusions from NLR analyses for OS and PFS. Therefore, we chose to conduct a subgroup analysis of follow-up ≥12 and <12 months. In both groups, a significant association was found between high NLR and worse OS and PFS prognosis. We attempted to conduct a similar analysis of follow-up ≥180 and <180 days; however, the results were similarly significant for both groups, and we did not include this analysis in our data. These findings highlighted that our findings are consistent and that studies with short follow-up may not have interfered with our results. We also chose to perform subgroup analyses of multivariate studies for OS and PFS. Every multivariate analysis displayed results consistent with our main findings that high NLR and PLR are associated with shorter OS and PFS. The results can be seen in Tables 2-5.
These consistent results favor an implementation of both biomarkers as possible tools to assess prognosis in advanced GC patients treated with immune-checkpoint blockade. However, we don’t believe that NLR and PLR are yet to be relied as a decision-making tool to indicate Immunotherapy based on their baseline values. We expect that new, larger studies will possibly validate this role in the future.
Previous studies have also investigated NLR role in prognosticating survival in early stage and locally advanced GC. A 2021 Italian study with a cohort of locally advanced patients treated with neoadjuvant chemotherapy demonstrated that baseline high NLR is significantly correlated with worse OS and PFS (3). A 2022 study also investigated patients with locally advanced disease receiving preoperative chemotherapy, finding a significant association between higher values of NLR and decreased OS (68). These results are in concordance with our findings and suggest the utility of novel biomarkers to prognosticate survival across the landscape of GC scenarios.
The present study is not the first systematic review to investigate the prognostic use of novel inflammatory markers in cancer. In 2018, a Chinese meta-analysis explored the significance of NLR in predicting OS and PFS prognosis among advanced cancer patients with various malignancies, such as renal cell, hepatocellular, and colorectal cancers, who underwent immunotherapy. It was concluded that NLR was a prognostic factor for both types of survival, although with high levels of heterogeneity (67). Similarly, two meta-analyses investigating PLR use to prognosticate survival in lung cancer patients treated with immune checkpoint blockade revealed significant associations between high PLR and worse survival outcomes. Taken together, these findings highlight the potential role of emerging biomarkers in cancer management in the era of immunotherapy. Complete blood counts and their derived novel inflammatory markers have been ruled cost-effective tools in several instances (69,70). Their straightforward application and affordability support their adoption, particularly when their ability to predict treatment prognosis has been validated. This is especially valid when prices for medications can exceed tens of thousands of US$ per year as is the case for immunotherapy (71-73).
Some issues, however, raise questions regarding the implementation of NLR and PLR in clinical practice. The first issue relates to the potential influence of other comorbidities on NLR and PLR levels, which could interfere with their ability to be used in prognosticating cancer. The influence of other concomitant diseases on biomarker levels has not been studied, and an investigation into this topic is timely and appropriate. Another question that arises is the current scarce understanding regarding dynamics between neutrophils, platelets, lymphocytes, and the tumor microenvironment, as their interaction is complex and intricate. This lack of knowledge contributes to the unknown variation in NLR and PLR levels. Currently, there is a lack of studies dedicated to thoroughly investigating the dynamics of novel inflammatory markers, and research efforts in this direction are needed.
The wide range of cutoff values across studies is another point of contention when considering NLR and PLR as biomarkers for prognosis in oncology. This divergence is, in part, a consequence of the diverse methods employed by studies with respect to cutoff points. Currently, there is no standardized value that can be used across studies. We conducted subgroup analysis investigating cutoffs ≥3 and <3 for NLR and >200 and ≤200 for PLR, with all analyses resulting in a significant association between high PLR and poor OS and PFS. The exact cutoff values for NLR and PLR remain unknown. A multicenter and international task to determine standardized cutoffs for each biomarker is needed to align conclusions of different studies around one common cutoff value.
Our meta-analysis possesses some limitations. First, our study encompassed a mixture of studies, with a combination of prospective and retrospective designs, thereby introducing a risk of bias, especially for the PLR analysis. Second, as previously highlighted, there is a diversity of cutoff values across studies, and definitive cutoff values could not be determined. Furthermore, we had a high risk of publication bias in our NLR analysis for OS. Additionally, we pooled data from multivariate and univariate models.
Despite these considerations, we believe that our meta-analysis boasts several positive aspects. The present systematic review exclusively included high-quality studies. Moreover, we had a remarkable heterogeneity of 0% in most of the PLR assessments, which reduced the risk of bias. Furthermore, although most studies related to our topic were conducted in Asia, we included one conducted in Europe, diversifying the population.
Conclusions
In conclusion, an elevated NLR has been demonstrated to have significant correlations with unfavorable OS and PFS outcomes among patients with advanced GC/GEJC undergoing immunotherapy. These findings underscore its potential utility as an accessible biomarker for prognostic assessment in the era of immunotherapy. Elevated PLR has also been shown to have significant associations with shortened OS and PFS. However, careful consideration should be given to this finding, as the data for PLR primarily consist of retrospective studies with small sample sizes. Additional prospective studies, as well as research delving into the interactions between neutrophils, platelets, lymphocytes, and the microenvironment, are further needed to validate our findings regarding both biomarkers.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-808/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-808/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-808/coif). P.N.A. Jr received lecture honoraria from Aché, Amgen, AstraZeneca, Astellas, Bayer, Boehringer Ingelheim, Gilead, GSK, Merck Co, Sanofi, Servier, and United Medical; received Advisory Board Fee from Adium, Gilead, and Daiichi Sankyo; and received support for attending a meeting from AstraZeneca. None of them are related to this manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol 2022;28:1187-203. [Crossref] [PubMed]
- Machlowska J, Baj J, Sitarz M, et al. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci 2020;21:4012. [Crossref] [PubMed]
- Zurlo IV, Schino M, Strippoli A, et al. Predictive value of NLR, TILs (CD4+/CD8+) and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Cancer Immunol Immunother 2022;71:45-55. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021;600:727-30. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:167-92. [Crossref] [PubMed]
- Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:234-47. [Crossref] [PubMed]
- Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond) 2021;41:747-95. [Crossref] [PubMed]
- Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023;26:1-25.
- Kim TH, Kim IH, Kang SJ, et al. Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach. J Gastric Cancer 2023;23:3-106. [Crossref] [PubMed]
- Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:1005-20. [Crossref] [PubMed]
- Xiang J, Gong W, Sun P, et al. Efficacy and safety of camrelizumab plus chemotherapy versus chemotherapy alone in patients with untreated, HER2-negative, unresectable locally advanced, or metastatic gastric cancer or gastroesophageal junction cancer: a retrospective comparative cohort study. J Gastrointest Oncol 2022;13:2874-84. [Crossref] [PubMed]
- Voutsadakis IA. A Systematic Review and Meta-analysis of PD-1 and PD-L1 Inhibitors Monotherapy in Metastatic Gastric and Gastroesophageal Junction Adenocarcinoma. Euroasian J Hepatogastroenterol 2020;10:56-63. [Crossref] [PubMed]
- Ma S, Chen F. Common strategies for effective immunotherapy of gastroesophageal cancers using immune checkpoint inhibitors. Pathol Res Pract 2022;238:154110. [Crossref] [PubMed]
- Wang F, Wei XL, Wang FH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol 2019;30:1479-86. [Crossref] [PubMed]
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021;71:264-79. [Crossref] [PubMed]
- Naseem M, Barzi A, Brezden-Masley C, et al. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat Rev 2018;66:15-22. [Crossref] [PubMed]
- Kanda T, Yajima M, Ikuta K. Epstein-Barr virus strain variation and cancer. Cancer Sci 2019;110:1132-9. [Crossref] [PubMed]
- Li J, Liu H, Liu W, et al. Predicting gastric cancer tumor mutational burden from histopathological images using multimodal deep learning. Brief Funct Genomics 2023; Epub ahead of print. [Crossref] [PubMed]
- Sun K, Jia K, Lv H, et al. EBV-Positive Gastric Cancer: Current Knowledge and Future Perspectives. Front Oncol 2020;10:583463. [Crossref] [PubMed]
- Choi YY, Bae JM, An JY, et al. Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta-analysis. J Surg Oncol 2014;110:129-35. [Crossref] [PubMed]
- Liang T, Chen J, Xu G, et al. Platelet-to-Lymphocyte Ratio as an Independent Factor Was Associated With the Severity of Ankylosing Spondylitis. Front Immunol 2021;12:760214. [Crossref] [PubMed]
- El-Gazzar AG, Kamel MH, Elbahnasy OKM, et al. Prognostic value of platelet and neutrophil to lymphocyte ratio in COPD patients. Expert Rev Respir Med 2020;14:111-6. [Crossref] [PubMed]
- Yang AP, Liu JP, Tao WQ, et al. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol 2020;84:106504. [Crossref] [PubMed]
- Jiang M, Peng W, Pu X, et al. Peripheral Blood Biomarkers Associated With Outcome in Non-small Cell Lung Cancer Patients Treated With Nivolumab and Durvalumab Monotherapy. Front Oncol 2020;10:913. [Crossref] [PubMed]
- Park CK, Oh HJ, Kim MS, et al. Comprehensive analysis of blood-based biomarkers for predicting immunotherapy benefits in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res 2021;10:2103-17. [Crossref] [PubMed]
- Wang DS, Ren C, Qiu MZ, et al. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour Biol 2012;33:749-56. [Crossref] [PubMed]
- Lin JX, Wang ZK, Huang YQ, et al. Dynamic Changes in Pre- and Postoperative Levels of Inflammatory Markers and Their Effects on the Prognosis of Patients with Gastric Cancer. J Gastrointest Surg 2021;25:387-96. [Crossref] [PubMed]
- Huang Z, Fu Z, Huang W, et al. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med 2020;38:641-7. [Crossref] [PubMed]
- Kong W, He Y, Bao H, et al. Diagnostic Value of Neutrophil-Lymphocyte Ratio for Predicting the Severity of Acute Pancreatitis: A Meta-Analysis. Dis Markers 2020;2020:9731854. [Crossref] [PubMed]
- Li S, Liu Y, Liu S, et al. Predictive Values of Inflammation-Related Markers and Thyroid Function in Pediatric Thyroid Cancer Patients. Front Pediatr 2021;9:802214. [Crossref] [PubMed]
- Simonaggio A, Elaidi R, Fournier L, et al. Variation in neutrophil to lymphocyte ratio (NLR) as predictor of outcomes in metastatic renal cell carcinoma (mRCC) and non-small cell lung cancer (mNSCLC) patients treated with nivolumab. Cancer Immunol Immunother 2020;69:2513-22. [Crossref] [PubMed]
- Chen W, Xin S, Xu B. Value Research of NLR, PLR, and RDW in Prognostic Assessment of Patients with Colorectal Cancer. J Healthc Eng 2022;2022:7971415. [Crossref] [PubMed]
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [Crossref] [PubMed]
- Formica V, Morelli C, Patrikidou A, et al. Gastric Inflammatory Prognostic Index (GIPI) in Patients with Metastatic Gastro-Esophageal Junction/Gastric Cancer Treated with PD-1/PD-L1 Immune Checkpoint Inhibitors. Target Oncol 2020;15:327-36. [Crossref] [PubMed]
- Sakai D, Omori T, Fumita S, et al. Real-world effectiveness of third- or later-line treatment in Japanese patients with HER2-positive, unresectable, recurrent or metastatic gastric cancer: a retrospective observational study. Int J Clin Oncol 2022;27:1154-63. [Crossref] [PubMed]
- Suzuki H, Yamada T, Sugaya A, et al. Retrospective analysis for the efficacy and safety of nivolumab in advanced gastric cancer patients according to ascites burden. Int J Clin Oncol 2021;26:370-7. [Crossref] [PubMed]
- Tanaka K, Tanabe H, Sato H, et al. Prognostic factors to predict the survival in patients with advanced gastric cancer who receive later-line nivolumab monotherapy-The Asahikawa Gastric Cancer Cohort Study (AGCC). Cancer Med 2022;11:406-16. [Crossref] [PubMed]
- Wan M, Ding Y, Mao C, et al. Association of inflammatory markers with survival in patients with advanced gastric cancer treated with immune checkpoint inhibitors combined with chemotherapy as first line treatment. Front Oncol 2022;12:1029960. [Crossref] [PubMed]
- Yamada T, Hayashi T, Inokuchi Y, et al. Impact of the Neutrophil-to-Lymphocyte Ratio on the Survival of Patients with Gastric Cancer Treated with Nivolumab Monotherapy. Target Oncol 2020;15:317-25. [Crossref] [PubMed]
- Hayano K, Ohira G, Kano M, et al. Prognostic Impact of Hepatic Steatosis Evaluated by CT on Immunotherapy for Gastric Cancer: Associations with Sarcopenia, Systemic Inflammation, and Hormones. Oncology 2023;101:185-92. [Crossref] [PubMed]
- Gou M, Zhang Y. Pretreatment platelet-to-lymphocyte ratio (PLR) as a prognosticating indicator for gastric cancer patients receiving immunotherapy. Discov Oncol 2022;13:118. [Crossref] [PubMed]
- Chen J, Wu X, Zhu S, et al. Changes in Neutrophil to Lymphocyte Ratio, Lymphocyte to Monocyte Ratio, and Platelet to Lymphocyte Ratio During Palliative Radiotherapy May Predict Efficacy of Immune Checkpoint Inhibitor as Re-Challenge Treatment in Advanced Gastric Cancer: A Case Report. Front Oncol 2022;12:873213. [Crossref] [PubMed]
- Gou M, Qu T, Wang Z, et al. Neutrophil-to-Lymphocyte Ratio (NLR) Predicts PD-1 Inhibitor Survival in Patients with Metastatic Gastric Cancer. J Immunol Res 2021;2021:2549295. [Crossref] [PubMed]
- Kim JH, Ryu MH, Park YS, et al. Predictive biomarkers for the efficacy of nivolumab as ≥3(rd)-line therapy in patients with advanced gastric cancer: a subset analysis of ATTRACTION-2 phase III trial. BMC Cancer 2022;22:378. [Crossref] [PubMed]
- Li T, Liu T, Zhao L, et al. Effectiveness and safety of anti-PD-1 monotherapy or combination therapy in Chinese advanced gastric cancer: A real-world study. Front Oncol 2023;12:976078. [Crossref] [PubMed]
- Namikawa T, Yokota K, Tanioka N, et al. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today 2020;50:1486-95. [Crossref] [PubMed]
- Ogata T, Satake H, Ogata M, et al. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget 2018;9:34520-7. [Crossref] [PubMed]
- Ota Y, Takahari D, Suzuki T, et al. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother Pharmacol 2020;85:265-72. [Crossref] [PubMed]
- Qu Z, Wang Q, Wang H, et al. The Effect of Inflammatory Markers on the Survival of Advanced Gastric Cancer Patients Who Underwent Anti-Programmed Death 1 Therapy. Front Oncol 2022;12:783197. [Crossref] [PubMed]
- Ruan DY, Chen YX, Wei XL, et al. Elevated peripheral blood neutrophil-to-lymphocyte ratio is associated with an immunosuppressive tumour microenvironment and decreased benefit of PD-1 antibody in advanced gastric cancer. Gastroenterol Rep (Oxf) 2021;9:560-70. [Crossref] [PubMed]
- Zhang S, Qiu C, Yu H, et al. Prognostic value of neutrophil to lymphocyte ratio in gastric cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol 2023;13:1070019. [Crossref] [PubMed]
- Li LL, Pan LS. Prognostic value of neutrophil-to-lymphocyte ratio in gastric cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Kaohsiung J Med Sci 2023;39:842-52. [Crossref] [PubMed]
- Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 2013;33:S79-84. [Crossref] [PubMed]
- Bonecchi R, Borroni EM, Anselmo A, et al. Regulation of D6 chemokine scavenging activity by ligand- and Rab11-dependent surface up-regulation. Blood 2008;112:493-503. [Crossref] [PubMed]
- Granot Z, Fridlender ZG. Plasticity beyond cancer cells and the "immunosuppressive switch". Cancer Res 2015;75:4441-5. [Crossref] [PubMed]
- Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol 2017;102:343-9. [Crossref] [PubMed]
- Wang TT, Zhao YL, Peng LS, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut 2017;66:1900-11. [Crossref] [PubMed]
- Pirozzolo G, Gisbertz SS, Castoro C, et al. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: a systematic review and meta-analysis. J Thorac Dis 2019;11:3136-45. [Crossref] [PubMed]
- Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol 2018;11:125. [Crossref] [PubMed]
- Lazar S, Goldfinger LE. Platelets and extracellular vesicles and their cross talk with cancer. Blood 2021;137:3192-200. [Crossref] [PubMed]
- Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol 2019;234:8509-21. [Crossref] [PubMed]
- Jin H, Zhang G, Liu X, et al. Blood neutrophil-lymphocyte ratio predicts survival for stages III-IV gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol 2013;11:112. [Crossref] [PubMed]
- Cho IR, Park JC, Park CH, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer 2014;17:703-10. [Crossref] [PubMed]
- Murakami Y, Saito H, Shimizu S, et al. Neutrophil-to-Lymphocyte Ratio as a Prognostic Indicator in Patients With Unresectable Gastric Cancer. Anticancer Res 2019;39:2583-9. [Crossref] [PubMed]
- Aldemir MN, Turkeli M, Simsek M, et al. Prognostic Value of Baseline Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios in Local and Advanced Gastric Cancer Patients. Asian Pac J Cancer Prev 2015;16:5933-7. [Crossref] [PubMed]
- Jiang T, Qiao M, Zhao C, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: a meta-analysis. Cancer Immunol Immunother 2018;67:713-27. [Crossref] [PubMed]
- Tomás TC, Eiriz I, Vitorino M, et al. Neutrophile-to-lymphocyte, lymphocyte-to-monocyte, and platelet-to-lymphocyte ratios as prognostic and response biomarkers for resectable locally advanced gastric cancer. World J Gastrointest Oncol 2022;14:1307-23. [Crossref] [PubMed]
- Hamed MO, Roberts KJ, Smith AM, et al. Elevated pre-operative neutrophil to lymphocyte ratio predicts disease free survival following pancreatic resection for periampullary carcinomas. Pancreatology 2013;13:534-8. [Crossref] [PubMed]
- Sayed AA. The Cost-Effectiveness of Requesting a Complete Blood Count (CBC) in the Management of COVID-19 in Saudi Arabia. Healthcare (Basel) 2022;10:1780. [Crossref] [PubMed]
- Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer 2018;6:128. [Crossref] [PubMed]
- Shu Y, Ding Y, Zhang Q. Cost-Effectiveness of Nivolumab Plus Chemotherapy vs. Chemotherapy as First-Line Treatment for Advanced Gastric Cancer/Gastroesophageal Junction Cancer/Esophagel Adenocarcinoma in China. Front Oncol 2022;12:851522. [Crossref] [PubMed]
- Marupuru S, Arku D, Axon DR, et al. Cost-effectiveness analysis of nivolumab-chemotherapy as first-line therapy for locally advanced/metastatic gastric cancer: a United States payer perspective. Expert Rev Pharmacoecon Outcomes Res 2023;23:831-41. [Crossref] [PubMed]


