
Circulating tumor DNA analysis guiding adjuvant treatment in resected stage III cholangiocarcinoma: a case report
Highlight box
Key findings
• Detecting early recurrence after resection in cholangiocarcinoma (CCA) using circulating tumor DNA (ctDNA) has the potential to alter surveillance or adjuvant treatment plan.
What is known and what is new?
• Recent studies have revealed the promising utility of ctDNA as a tool for monitoring treatment response and early recurrence detection in other solid tumors. However, there is a paucity of data for ctDNA in CCA.
• This case report demonstrates the potential utility of ctDNA in CCA as (I) an early detection tool for recurrence and (II) a response monitoring tool that can optimize therapy.
What is the implication, and what should change now?
• One may consider escalating therapy, changing therapy or increasing the frequency of radiographic surveillance when ctDNA becomes positive during surveillance in CCA. Larger prospective studies are needed to establish the role of ctDNA in CCA.
Introduction
Cholangiocarcinoma (CCA) is a rare and aggressive gastrointestinal cancer. Unfortunately, 60–70% of early-stage CCA patients experience disease recurrence even with curative resection followed by standard capecitabine adjuvant therapy (1). Once the disease becomes unresectable, the prognosis is extremely poor, even with recent advances in systemic therapy for CCA (2,3), which shows the importance of finding a novel surveillance tool for early identification of relapse to enable early intervention and promote better outcomes. Currently, there is no reliable tool to identify CCA recurrence before radiographic detection.
In the current renaissance of circulating tumor DNA (ctDNA) in the field of oncology, various clinical applications of ctDNA have been proposed. Numerous recent studies from other solid tumors revealed ctDNA as a promising tool for monitoring of treatment response and for early recurrence detection (4). Longitudinal monitoring of ctDNA has shown high sensitivity and specificity, with molecular identification of relapse 3–6 months prior to conventional surveillance in other solid tumors (5-7). Based on recent promising evidence, ctDNA has been actively used in the real world as an optimizing tool for adjuvant therapy and for the early detection of recurrence in colorectal cancer (CRC) after curative resection (8-10). However, there is a paucity of data for ctDNA in CCA after curative surgery. In this report, we present a case with CCA that clearly demonstrated the various utilities of a personalized tumor-informed ctDNA assay for (I) early identification of recurrence; (II) aiding in prompt optimization of therapy by monitoring treatment response; and (III) early therapeutic intervention with immunotherapy or targeted therapies based on ctDNA that can potentially be translated into better survival outcomes. We present this article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-815/rc).
Case presentation
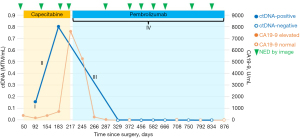
An 81-year-old male presented with jaundice and pruritis. Lab revealed hyperbilirubinemia and following computed tomography (CT) abdomen/pelvis revealed a 5.2 cm × 5.3 cm × 4.0 cm mass in the left hepatic lobe. He received endoscopic retrograde cholangiopancreatography-guided biliary stent placement with endoscopic ultrasound-guided biopsy of the liver lesion. The following pathology confirmed intrahepatic CCA. The subsequent positron emission tomography (PET)-CT redemonstrated the left hepatic lobe mass with hypermetabolic uptake with no evidence of other lesions or distance metastasis. The patient underwent left hepatic lobectomy, cholecystectomy, eight lymph node dissection, and Roux-en-Y hepaticojejunostomy. Following pathologic analysis revealed stage 3A (pT3, pN0) grade 2 moderately differentiated intrahepatic CCA with R0 resection and extensive lymphovascular and perineural invasion. On post-operative day (POD) 30, a following CT scan showed no evidence of disease (NED), and the patient was started on standard adjuvant capecitabine on POD 50.
On POD 92, we checked the tumor-informed multiplex polymerase chain reaction (PCR)-NGS assay (SignateraTM, Natera, Inc., Austin, TX, USA) which revealed an elevated ctDNA level [0.16 mean tumor molecule (MTM)/mL], indicating molecular residual disease (MRD)-positive. Following ctDNA stayed positive with increasing titer at 0.80 MTM/mL on POD 183, despite being on capecitabine. On POD 198, we checked the commercially available molecular profiling and next-generation sequencing (NGS) panels (Caris Life Sciences, Phoenix, AZ, USA) from the obtained liver specimen from resection, which revealed microsatellite instability-high (MSI-H) with tumor mutational burden-high (TMB-H, 21 mut/Mb) without actionable mutation. Carbohydrate antigen 19-9 (CA19-9) also continued to increase up to 7,594.9 U/mL on POD 217, from 175.6 U/mL on POD 92. Notably, surveillance CT scans kept NED on POD 126, 186, and 211, respectively. We had multiple extensive discussions with the patient in regard to rising ctDNA titer and CA19-9 in the setting of persistent radiographic remission while being on capecitabine for nearly 6 months. The patient was informed that generally speaking, ctDNA is a reliable predictor of cancer recurrence, and the rise in titer indicated that his tumor was most likely resistant to current adjuvant treatment.
Given the tumor is MSI-H, and the preferred toxicity profile compared to the front-line chemotherapy option for CCA, we started pembrolizumab on POD 224 instead of close surveillance. The patient tolerated pembrolizumab well without noticeable side effects. ctDNA was cleared and converted to negative with pembrolizumab on POD 329. CA19-9 decreased and entered the reference range on POD 372. As of the last follow-up on POD 876, the patient has continued on pembrolizumab without noticeable side effects, and images continue to be NED with persistent negative MRD by ctDNA and normal CA19-9 level. Figure 1 visualized the clinical course of the case.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
To our knowledge, there has been no published data regarding the utility of tumor-informed ctDNA testing-based MRD detection (ctDNA-MRD) in CCA. This is the first case which clearly demonstrated the prompt optimization of therapy based on ctDNA-MRD in CCA. The case contains multiple clinical applications of ctDNA in CCA, including (I) early identification of recurrence; (II) monitoring treatment response by dynamics of ctDNA; and (III) early intervention of therapy by positive ctDNA-MRD that can potentially promote better survival outcomes.
Compared to tumor-agnostic ctDNA, tumor-informed ctDNA is considered more specific to target malignancy by filtering out clonal hematopoiesis of indeterminate potential (CHIP), which can be detected from non-malignant cells (5). The tumor-informed ctDNA assay is known to indicate false positives extremely rarely, and showed high specificity of up to 100% for recurrence, with promising positive predictive value up to 100% in other solid tumors (4,11-13). In addition, numerous studies demonstrated that the longitudinal ctDNA check enables early detection of recurrence during surveillance after curative resection in other solid tumors (5-7,9-11). Based on this evidence, we assumed the patient had recurrent disease without radiographic relapse. We offered two options to the patient, with their full understanding that there was no prospective data demonstrating any superior survival benefits for either course: (I) close radiographic monitoring while withholding new treatment, or (II) starting early intervention before radiographic recurrence. This would be a common dilemma at a clinic in the real world, with ctDNA-MRD positive during surveillance. Our patient and team decided to initiate a new systemic therapy before radiographic recurrence, namely pembrolizumab, to promote better outcomes.
An increasing body of evidence supports ctDNA dynamics as a surrogate biomarker for treatment response and survival outcomes in other solid tumors (4). A recent biomarker study from the IMvigor010 trial revealed that urothelial carcinoma patients treated with adjuvant atezolizumab who cleared ctDNA had superior survival outcomes compared to patients who remained positive for ctDNA (14). Similar results, namely ctDNA clearance followed by later improved radiologic response and lack of ctDNA clearance followed by a poor radiographic response and further survival endpoints, were observed in other solid tumors (15,16). Our case clearly demonstrated the differences in ctDNA dynamics between capecitabine and pembrolizumab. ctDNA was not cleared but titer increased even after near completion of the standard 6 months adjuvant capecitabine, which implies poor response and persistent MRD with high risk of radiographic recurrence. Conversely, under the pembrolizumab, ctDNA was cleared and continued to be negative. This reflects a deep molecular response to pembrolizumab that is well supported by radiographical NED for nearly 2 years. This finding implies we can optimize the therapeutic agent based on the ctDNA value as a surrogate biomarker prior to radiographic response.
MSI-H is relatively rare with around 3–10% in CCA (17,18). Pembrolizumab monotherapy showed a complete response rate of 9% with a median progression-free survival of 4.2 months in MSI-H CCA (19). In our case, the patient has been in radiographic and molecular remission for almost 2 and 1.6 years, respectively. This observed long survival implies the early initiation of treatment by ctDNA positive prior to radiographic recurrence can potentially add survival benefits compared to late initiation of treatment due to waiting until radiographic recurrence. This prompt optimization strategy of therapeutic agents based on the dynamics of ctDNA can potentially be applicable in other actionable mutations for CCA such as FGFR2 fusion, IDH1, and BRAF V600E mutation, though further verifications are needed for the clinical benefit of agents targeting those mutations in an adjuvant setting. Further verification of this concept should be followed in studies with larger populations. In fact, there are numerous ongoing randomized clinical trials seeking to verify this hypothesis that early therapeutic interventions by ctDNA-MRD positivity may have clinical benefits such as CIRCULATE and ALTAIR in CRC, and c-TRAK-TN in the breast (4).
Currently, except for capecitabine, none of the systemic agents are standard of care for adjuvant therapy in CCA. Positive results from ctDNA-MRD can pose dilemmas regarding whether to continue surveillance or start non-standard intervention which can potentially promote better outcomes. Based on established evidence from other solid tumors, the positive tumor-informed ctDNA assay is almost the definitive presence of surveillance-targeting malignancy (9). Therefore, considering additional adjuvant therapy on standard capecitabine can be a reasonable option for ctDNA-MRD-positive cases in resected CCA. However, there are risks of overtreatment and side effects with this non-standard ctDNA-guided escalation adjuvant strategy. Our case had MSI-H with TMB-H, for which a relatively low-risk intervention, namely pembrolizumab, helped clear the ctDNA, and the patient remained NED. With the same logic, cases with actionable mutations can be candidates for this ctDNA-guided escalation approach with targeted agents, which are also relatively low-risk interventions. This approach should be more cautious, especially for cases without actionable mutations, as the risk of overtreatment and side effects is higher with off-the-label cytotoxic chemotherapy agents compared to targeted agents or immune checkpoint inhibitors.
Conclusions
Our case demonstrated the potential utility of tumor-informed ctDNA in CCA as (I) an early detection tool prior to radiographic recurrence; (II) a response monitoring tool as a surrogate biomarker that can prompt optimization of therapy; and (III) showed that an early initiation of intervention with immunotherapy or potentially targeted agents based on ctDNA can possibly promote better survival outcomes. Since our study is limited to a single-patient longitudinal study, this alone cannot establish the above utilities of ctDNA in this rare tumor type. Thus, larger prospective studies are needed to establish the role of tumor-informed ctDNA assay in CCA.
Acknowledgments
We are deeply grateful to our patient, who allowed us to present his clinical information as a case report.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-815/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-815/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-815/coif). R.D.K. reports consulting/advisory fees from AstraZeneca, Exelixis, Ipsen, Eisai, Roche, and Pfizer and Speakers Bureau fees from Incyte and AstraZeneca. C.M.R. reports Speakers Bureau fees from AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bridgewater J, Fletcher P, Palmer DH, et al. Long-Term Outcomes and Exploratory Analyses of the Randomized Phase III BILCAP Study. J Clin Oncol 2022;40:2048-57. [Crossref] [PubMed]
- Oh DY, He AR, Qin S, et al. 78P Updated overall survival (OS) from the phase III TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+ GC) in patients (pts) with advanced biliary tract cancer (BTC). Ann Oncol 2022;33:S1462-3. [Crossref]
- Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023;401:1853-65. [Crossref] [PubMed]
- Kasi PM, Fehringer G, Taniguchi H, et al. Impact of Circulating Tumor DNA-Based Detection of Molecular Residual Disease on the Conduct and Design of Clinical Trials for Solid Tumors. JCO Precis Oncol 2022;6:e2100181. [Crossref] [PubMed]
- Routman DM, Chera BS, Gupta GP. Circulating Tumor DNA Biomarkers for Early Detection of Oligometastasis. Cancer J 2020;26:116-23. [Crossref] [PubMed]
- Garcia-Murillas I, Chopra N, Comino-Méndez I, et al. Assessment of Molecular Relapse Detection in Early-Stage Breast Cancer. JAMA Oncol 2019;5:1473-8. [Crossref] [PubMed]
- Chera BS, Kumar S, Shen C, et al. Plasma Circulating Tumor HPV DNA for the Surveillance of Cancer Recurrence in HPV-Associated Oropharyngeal Cancer. J Clin Oncol 2020;38:1050-8. [Crossref] [PubMed]
- Tie J, Cohen JD, Lahouel K, et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N Engl J Med 2022;386:2261-72. [Crossref] [PubMed]
- Kotani D, Oki E, Nakamura Y, et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med 2023;29:127-34. [Crossref] [PubMed]
- Oki E, Kotani D, Nakamura Y, et al. Circulating tumor DNA dynamics as an early predictor of recurrence in patients with radically resected colorectal cancer: Updated results from GALAXY study in the CIRCULATE- Japan. J Clin Oncol 2023;41:3521. [Crossref]
- Coombes RC, Page K, Salari R, et al. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clin Cancer Res 2019;25:4255-63. [Crossref] [PubMed]
- Reinert T, Henriksen TV, Christensen E, et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol 2019;5:1124-31. [Crossref] [PubMed]
- Azad TD, Chaudhuri AA, Fang P, et al. Circulating Tumor DNA Analysis for Detection of Minimal Residual Disease After Chemoradiotherapy for Localized Esophageal Cancer. Gastroenterology 2020;158:494-505.e6. [Crossref] [PubMed]
- Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021;595:432-7. [Crossref] [PubMed]
- Magbanua MJM, Swigart LB, Wu HT, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol 2021;32:229-39. [Crossref] [PubMed]
- Tie J, Kinde I, Wang Y, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol 2015;26:1715-22. [Crossref] [PubMed]
- Silva VW, Askan G, Daniel TD, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol 2016;5:62. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2020;38:1-10. [Crossref] [PubMed]


