Evolution of pancreatic surgery over time and effects of centralization—a single-center retrospective cohort study
Highlight box
Key findings
• We report improved long-term results, and high-quality short-term outcomes in a cohort of 566 consecutive pancreatic resections performed at a Northern Finnish tertiary center over twenty years.
What is known and what is new?
• Centralization of pancreatic surgery, improved oncological regimens, and better management of complications have reduced postoperative mortality and Clavien-Dindo III-V complications worldwide, while long-term survival has improved concurrently only slightly.
• As the hospital volume of our tertiary center has substantially grown, and operative indications have extended, the short-term outcomes have remained below benchmark levels and long-term survival has improved.
What is the implication, and what should change now?
• Careful perioperative care and sufficient operative volume enable good short-term results even with less fit patients, and after advanced pancreatic surgery. Developing preoperative assessment of patient fitness and tumor characteristics will still offer means to improve outcomes in the future.
Introduction
Pancreatic surgery carries a high rate of postoperative complications and morbidity (1). Improved prevention and management of complications have been achieved in recent decades, resulting in decreased postoperative mortality. The advances are due to centralization of pancreatic surgery (2,3), and concurrent progress in perioperative care, surgical techniques, and management of complications (4,5). Benchmark studies have attempted to define acceptable quality limits for postoperative mortality, morbidity, procedure-specific complications as well as health care process and structure related metrics (6,7).
Pancreatic ductal adenocarcinoma (PDAC) has an extremely poor prognosis—in all-stage patients, 5-year overall survival (OS) remains 6–10% (8,9). Among resectable patients, high-volume centers have reported 5-year survival rates ranging from 15% to 30% (10-14), while in nationwide register-based studies, 5-year survival reaches 17–22% (15,16). In the most recent two decades, prognosis of PDAC has slightly improved (17). Adjuvant chemotherapy, the standard of care after the randomized controlled trials in 2004 and 2007 (18,19), has been suggested as the main contributor to extended survival.
The aim of our study was to describe a cohort of 566 consecutive pancreatectomies from a twenty-year period. We analyzed the trends in short-term outcomes of all-cause pancreatic surgery and long-term survival of resected PDAC patients. We hypothesized that clinical advances and centralization would be reflected in the short- and/ or long-term results. We present the following article in accordance with the STROCSS reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-649/rc).
Methods
Study design
We designed a retrospective single center cohort study including all pancreatic resections (n=566) performed at Oulu University Hospital during years 2000–2020. Oulu University Hospital is the only tertiary center in Northern Finland. While the geographical capture region is vast, over 50% of Finland’s land area, the population of the catchment area is only 736,000. To analyze the evolution of single center pancreatectomies over two decades, we divided the cohort population into four subsets by time periods: years 2000–2005, 2006–2010, 2011–2015 and 2016–2020.
Data collection
The consecutive pancreatectomy patients were identified from the operational planning program. Clinical data for each patient were obtained from the medical records at Oulu University Hospital and the pathology reports from the archives of the Department of Pathology. The follow-up and death data were requested from the Statistics Finland’s Cause-of-Death Registry, and received until the end of 2019. Incidences of all-stage PDAC per each time quartile in Northern Finland were obtained from the Finnish Cancer Registry (FCR) for the period of 1st October 1999 to 31st December 2019.
Surgical treatment
The treatment of each tumor patient was planned at a multidisciplinary team (MDT) for gastrointestinal cancers. The treatment guidelines have been complied with the European Society for Medical Oncology guidelines until 2014 (20). Later, as neoadjuvant therapy for PDAC was introduced in our center, The National Comprehensive Cancer Network guidelines were applied (21). The pancreatectomy procedures were performed according to standardized techniques for pancreatoduodenectomy (PD) and left pancreatectomy (LP). PD technique included resecting distal stomach and pylorus, hepatico- and pancreaticojejunostomies on the same jejunal loop, and Roux-en-Y-reconstruction. Only duct-to-mucosa-anastomosing technique was applied. After initiating operations for borderline resectable pancreatic cancer (BRPC) patients, vascular resections were increasingly performed. In cases involving portomesenteric vein resections, the reconstructions were performed according to the size of the venous defect with venorraphy, patching, end-to-end anastomosis or by using an interposition graft (22). In cases where superior mesenteric artery involvement was suspected, a selected “artery first”-approach (23) was applied. LP included splenectomy, when indication for the operation was malignancy or suspicion of malignancy. Pancreas was routinely transected at the level of the pancreatic neck to minimize leakages (24,25). The closure of the pancreatic stump was in most cases performed using a stapler. Laparoscopic LP was introduced in 2009 at our center, and mainly chosen in cases with premalignant indication or small, contained malignancies.
Reported outcomes and guidelines
Reporting of the cohort study was structured according to the STROCSS 2021 Guidelines (26), and study was retrospectively registered (www.researchregistry.com, UIN: researchregistry7775). Comorbidity data was recorded according to the American Society of Anesthesiologists (ASA) Physical Classification, and the Royal College of Surgeons (RCS) Charlson Score, excluding pancreatic cancer (27).
General complications were recorded according to Clavien-Dindo classification (28). Chyle leaks, delayed gastric emptying (DGE), post-pancreatectomy hemorrhages and pancreatic fistulas were graded according to the International Study Group of Pancreatic Surgery (ISGPS) criteria (29-32). Reoperations were divided into laparotomy/laparoscopy, superficial wound revisions, and endoscopic procedures. Endovascular, and percutaneous procedures performed by interventional radiologists, were reported separately of reoperations. All postoperative survival times were defined as time from surgery to death from any cause or censoring at the time of last follow-up. The benchmark values for comparison were obtained from benchmark studies reviewed by Ou et al. (7).
The pathology reports were examined for basic histology of the specimen, TNM classification [American Joint Committee on Cancer, 8th edition (33)], tumor grade [WHO Classification of Tumors (34)], perineural, perivascular and lymphatic invasion, R status (R0 defined as >1 mm tumor free margin), and lymph node status.
Ethical statement
This study complied with the ethical standards of the Oulu University Institutional Research Committee and the Declaration of Helsinki (as revised in 2013). Data collection from the Finnish Cancer Registry was approved by the Finnish Social and Health Data Permit Authority (FINDATA, Dnro THL/3606/14.02.00/2020). The data collection was accepted by the Oulu University Hospital Ethics Committee (EETTMK: 81/2008) and by the National Supervisory Authority for Welfare and Health (VALVIRA; approval No. 10832/06.01.03.01/2014). Individual patient consent for this retrospective analysis was waived.
Statistics analysis
Statistics were performed to investigate the outcomes and trends over the study period in all-cause pancreatic surgery and curative-intent treatment of PDAC. Categorical variables were examined by applying the Chi square test, while continuous variables were compared by t-test or Mann-Whitney U test as appropriate. Numeric values are presented as medians (interquartile range). Survival was investigated by using the life table method and plotted with Kaplan-Maier curves. Survival rates were compared by the log-rank test. Cox’s regression model was used to analyze the relative risks of mortality and to obtain hazard ratios (HRs) with 95% confidence intervals (CIs) for 30-day, 90-day, 1-year and 3-year survival, by using three models. In the crude model no adjustment was performed. Model A was adjusted for potential confounding factors: age (continuous), sex (male or female), RCS (0, 1 or ≥2, excluding pancreatic cancer), preoperative CA19-9 level (continuous), pathological tumour stage (1, 2, 3 and 4), grade of differentiation (1, 2 and 3), neoadjuvant therapy (no or yes), and time period of surgery (2000–2005, 2006–2010, 2011–2015, and 2016–2020). Model B was adjusted for age (continuous), sex (male or female), RCS (0, 1 or ≥2, excluding pancreatic cancer), preoperative CA19-9 level (continuous), tumor size (mm, continuous), pathological tumor N-stage (0, 1 and 2), grade of differentiation (1, 2 and 3), neoadjuvant therapy (no or yes), and time period of surgery (2000–2005, 2006–2010, 2011–2015 and 2016–2020). For the analysis of 1- and 3-year survivals in the last time period (2016–2020), only patients operated on in 2016–2018, and 2016, respectively were included. Up to 20% (48/240) of the patient data were incomplete on tumor size, T- and N-stage, grade of differentiation, or CA19-9. Thus, both complete case analysis and multiple imputations were conducted. All confounding variables categorized as above, and all-cause mortality, were included as imputation variables. The number of imputed datasets was twenty. The fully conditional specification was used under the assumption, that the data were missing at random. Statistical analyses were performed using IBM SPSS version 27 (Armonk, NY, USA).
Results
Patients and operations
Median age of patients was 63 [57–71] years, and 49% [267] of patients were men. During years 2000–2020, a total of 566 pancreatectomies were performed at Oulu University Hospital. The cohort included 359 (63.0%) PDs, 130 (23.0%) open left pancreatectomies, 45 (8.0%) laparoscopic left pancreatectomies, 26 (5.1%) total pancreatectomies (TPs), and 6 (1.1%) enucleations. The most prevalent ASA classes were II (49.0%) and III (36.0%), while RCS was 0 in 43% of patients undergoing pancreatectomy. Duration of surgery was 8:20/3:50/9:10 hours for PD/LP/TP, and length of hospital stay after PD/LP/TP was 14/10/17 days, respectively. Patient- and operation-related characteristics are presented in Table 1, along with relevant benchmark reference rates.
Table 1
| Parameter | Values | Benchmark values |
|---|---|---|
| Age (years) [range] | 63 [57–71] | |
| Men, n (%) | 267 (49.0) | |
| BMI (kg/m2) [range] | 26 [23–29] | |
| Regular alcohol use, n (%) | 44 (7.7) | |
| Tobacco smoking, n (%) | 106 (19.0) | |
| ASA, n (%) | ||
| I | 39 (7.0) | |
| II | 263 (49.0) | |
| III | 195 (36.0) | |
| IV | 9 (1.7) | |
| N/A | 30 (5.2) | |
| RCS | ||
| 0 | 248 (43.0) | |
| 1 | 173 (30.0) | |
| 2 | 77 (113.0) | |
| ≥3 | 23 (4.0) | |
| N/A | 52 (9.1) | |
| Histology, n (%) | ||
| Pancreatic ductal adenocarcinoma | 230 (42.0) | |
| Periampullary adenocarcinoma | 27 (4.8) | |
| Cholangiocarcinoma | 35 (6.2) | |
| Duodenal adenocarcinoma | 13 (2.3) | |
| Neuroendocrine tumor | 61 (11.0) | |
| Cystic neoplasms | 67 (12.0) | |
| Other pancreatic carcinomas | 18 (3.2) | |
| Metastases | 24 (4.2) | |
| Benign, any | 71 (13.0) | |
| N/A | 10 (1.8) | |
| Operations, n (%) | ||
| PD | 359 (63.0) | |
| Open LP | 130 (23.0) | |
| Laparoscopic LP | 45 (8.0) | |
| Enucleation | 6 (1.1) | |
| TP | 26 (5.1) | |
| Preoperative biliary stenting, n (%) | 252 (49.0) | |
| Length of hospital stay (days) | ||
| PD | 14 [11–19] | ≤15 |
| Open LP | 10 [8.5–15] | |
| Laparoscopic LP | 10 [10–12] | |
| TP | 17 [17–18] | |
| All resections | 14 [10–18] | <21 |
| Duration of operation (h) | ||
| PD | 8.2 [7.3–9.3] | |
| Open LP | 3.5 [3.0–5.3] | |
| Laparoscopic LP | 3.5 [3.0–4.4] | |
| TP | 9.1 [9.3–10.4] | |
| All resections | 7.4 [5.3–9.1] | <10 |
Values are shown as n (%) or median [IQR]. BMI, body mass index; ASA, American Society of Anesthesiologists; N/A, not available; RCS, Royal College of Surgeons; PD, pancreatoduodenectomy; LP, left pancreatectomy; TP, total pancreatectomy.
Pathological characteristics
PDAC was the histological diagnosis in 42% [240] of cases. Other adenocarcinomas originating from distal bile duct, ampulla Vater or duodenum, mucinous cystic neoplasia (MCN) or intraductal mucinous neoplasia (IPMN carcinomas) were rare (Table 1) and excluded from PDAC analyses. According to PDAC pathology reports, T2 tumors were most frequent (61%), and lymph node metastases were present in most specimens (66%). Tumor cells were found within 1mm of resection margins (R1) in 38% of cases. Pathological diagnoses of resected specimens, tumor characteristics, and relevant benchmarking values are presented in Table 2.
Table 2
| Parameter | n (%) | Benchmark values |
|---|---|---|
| T, n (%) | ||
| 1 | 22 (9.2) | |
| 2 | 146 (61.0) | |
| 3 | 55 (23.0) | |
| 4 | 8 (3.3) | |
| N/A | 9 (9.8) | |
| N, n (%) | ||
| 0 | 70 (29.0) | |
| 1 | 96 (40.0) | |
| 2 | 63 (26.0) | |
| N/A | 11 (4.6) | |
| M, n (%) | ||
| 0 | 238 (99.0) | |
| 1 | 2 (1.0) | |
| Stage, n (%) | ||
| I | 62 (26.0) | |
| II | 101 (42.0) | |
| III | 69 (29.0) | |
| IV | 2 (1.0) | |
| N/A | 6 (2.5) | |
| Grade | ||
| 1 | 38 (16.0) | |
| 2 | 87 (36.0) | |
| 3 | 66 (28.0) | |
| N/A | 49 (20.0) | |
| Perineural invasion, n (%) | ||
| No | 30 (13.0) | |
| Yes | 118 (49.0) | |
| N/A | 92 (62.0) | |
| Perivascular invasion, n (%) | ||
| No | 68 (28.0) | |
| Yes | 55 (23.0) | |
| N/A | 117 (49.0) | |
| Perilymphatic invasion, n (%) | ||
| No | 67 (28.0) | |
| Yes | 80 (33.0) | |
| N/A | 93 (39.0) | |
| R status, n (%) | ||
| R0 | 114 (48.0) | |
| R1 | 92 (38.0) | ≤46 |
| N/A | 34 (14.0) | |
| Lymph nodes harvested, median [range] | 17 [0–55] | ≥15 |
Oncological treatment
Information on the implementation of adjuvant treatment was available only in 141 cases (61%), mostly due to loss of follow-up data on patients receiving adjuvant therapy at secondary hospitals (37%). Of the 141 patients followed, 102 (72%) were referred to adjuvant therapy. Neoadjuvant chemotherapy was administered to 28 patients (12%).
Short-term outcomes
Clavien-Dindo (I–V) complications in all pancreatectomies amounted to 325/566 patients (57.4%). Patients suffered severe Clavien-Dindo III–V complications in 145 (25.6%) cases (Table 3). Grade B–C fistulas occurred in 8.9% of PDs, and in 9.1% of left pancreatectomies. Grade A–C bile leakages were seen in 4.0% of cases after PD or TP. Grade A–C DGE was found in 13%, and grade A–C hemorrhages in 6.9% of all pancreatectomies. Total amount of reoperations was 95/566 (16.8%), including 50 (8.8%) relaparotomies, 19 (3.4%) superficial wound revisions, and 26 (4.6%) endoscopic procedures. The most common indications for relaparotomy were postoperative fascial dehiscence (16/50), followed by leakage of any of the anastomoses (11/50), postpancreatectomy hemorrhage (9/50), and colonic ischemia (6/50). Postoperative interventional radiology was required in 12.9% of patients. Endovascular techniques or percutaneous biliary procedures were employed in 1.4%, and image-guided drainage or needle aspiration in 11.5% of cases. Readmission rate was 7.8% within 30 days, and 11.3% within 90 days. 30- and 90-day mortality rates were 1.0% and 1.4%, respectively. The complication profile of all pancreatectomies and the benchmarking reference rates are presented in Table 3.
Table 3
| Parameter | Oulu University Hospital, n (%) | Benchmark cutoff % (6,7,34) |
|---|---|---|
| Clavien-Dindo | ||
| I–II | 180 (32.0) | ≤62 |
| IIIa | 83 (15.0) | |
| IIIb | 23 (4.1) | ≤30 |
| IV | 32 (5.7) | |
| V | 7 (1.2) | ≤5 |
| III–V | 145 (25.6) | |
| Total | 325 (57.0) | ≤73 |
| 30-day mortality | 5 (0.9) | |
| 90-day mortality | 8 (1.4) | ≤1.6 |
| PD related mortality | 4 (1.1) | |
| LP related mortality | 3 (1.7) | |
| TP related mortality | 0 (0.0) | |
| Pancreatic fistula B–C rate | ||
| PD | 32 (8.9) | ≤19 |
| LP | 16 (9.1) | |
| Type of complication | ||
| Pancreatic fistula (grade A–C) | 72/540 (13.0) | <31 |
| Biliary fistula (grade A–C) | 15 (4.0) | <14 |
| Hemorrhage (grade A–C) | 39 (6.9) | <21 |
| DGE (grade A–C) | 72 (13.0) | |
| Chyle leak (A–C) | 48 (8.5) | |
| Reoperations | ||
| Relaparotomy or laparoscopy | 50 (8.8) | |
| Subcutaneous wound revision | 19 (3.4) | |
| Endoscopic procedures | 26 (4.6) | |
| Total | 95 (16.8) | <20 |
| Radiological interventions | ||
| Endovascular procedures | 8 (1.4) | |
| Percutaneous procedures | 65 (11.5) | |
| Total | 73 (12.9) | |
| Readmissions | ||
| 30-day | 44 (7.8) | |
| 90-day | 64 (11.3) | <21 |
PD, pancreaticoduodenectomy; LP, left pancreatectomy; TP, total pancreatectomy; DGE, delayed gastric emptying.
Long-term outcomes of PDAC patients
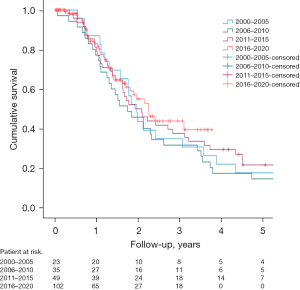
The median follow-up of patients with PDAC was 23 [13–46] months. The overall 1-, 3-, and 5-year survival rates of PDAC patients operated on during years 2000–2020 are shown in Figure 1 and Table 4. The median OS in the entire cohort was 25 (21.7–28.3) months. In crude or adjusted multivariate models with multiple imputations, no improvement in 3-year, 1-year, 90-day, and 30-day prognosis were found (data not shown). Resection rate of PDAC patients for the entire study period was 7.1%.
Table 4
| Time quartile | 1-year (%) | 3-year (%) | 5-year (%) |
|---|---|---|---|
| 2000–2005 | 87.0 | 34.8 | 17.4 |
| 2006–2010 | 77.1 | 31.4 | 14.3 |
| 2011–2015 | 79.6 | 36.7 | 21.4 |
| 2016–2020 | 82.2 | 39.4 | – |
PDAC, pancreatic ductal adenocarcinoma.
Time trends
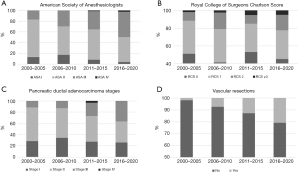
Patient and tumor related characteristics were compared between the four time periods. Patients’ median age, ratio between sexes, and BMI showed no changes. The distribution of different procedures remained constant. Patients with ASA class III were more frequently operated on in 2011–2015 (31%) and 2016–2020 (46%), than in earlier years (18%), whereas patients with ASA class I represented only 4% of pancreatectomy patients in 2016–2020 vs. 9% in 2000–2005. RCS of operated patients, however, showed no significant changes during the entire study period (Figure 2A,2B). Pathological staging of PDAC patients showed an increase in the resected stage III diseases in the last two time periods. A corresponding trend could be observed in the increased frequency of vascular resections (Figure 2C,2D).
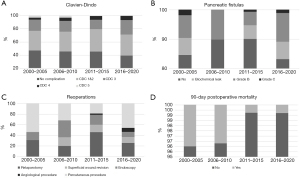
Short- and long-term outcomes were compared between time periods. Clavien-Dindo (I–V) complication rates after pancreatectomies remained mostly constant (Figure 3A). Mean duration of surgery was 6:50 hours in 2000–2015 but grew substantially to 8:11 hours in 2016–2020. Clinically relevant grade B–C fistula rates varied from 7% to 17% between time periods, but with no significant differences or trends (Figure 3B). Interventions were however performed at an increasing rate. Radiological intervention rate rose from 9.3% in 2000–2010 to 14.3% in 2011–2020. Endovascular techniques were only employed during the latest time period, to 7/266 (2.6%) patients. Reoperation rate showed a notable increase from 9.4% in 2000–2005 to 16.2% in 2016–2020 (Figure 3C). Post-operative mortality rates remained low throughout the study period, but 90-day mortality decreased from 3.1% in 2000–2010 to 0.74% in 2011–2020 (Figure 3D). Long-term OS (5-year) of PDAC patients improved from 14.3% in 2006–2011 to 21.4% in 2011–2015, and similarly the 3-year OS showed an increasing trend between years 2006–2011 and 2016–2020 (31.4% vs. 39.4%). However, statistical significance was not reached. Kaplan-Meier curves by time quartiles, and the overall 1-, 3- and 5-year survival rates are shown in Figure 1 and Table 4.
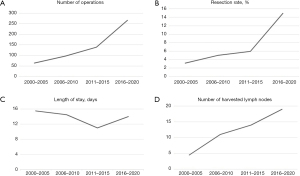
Health care process related metrics were also evaluated. Hospital volume grew significantly during twenty years from 67 in 2000–2005 to 266 in 2016–2020, and a simultaneous rise in the resection rate of PDAC was observed, especially during the last time period (Figure 4A,4B). Length of hospital stay shortened, with clinical significance only, from 16 to 14 days during the study period (Figure 4C). R status of surgical margins showed no relevant changes. Number of harvested lymph nodes increased significantly from 4 in 2000–2005 (IQR, 1.0–14.0) to 19 (9.0–24.0) in the two last time quartiles (Figure 4D). Referral rate to adjuvant therapy improved significantly from 20% in 2000–2005, to over 80% in the last three quartiles. The actual administration of adjuvant treatment reached 84% at Oulu University Hospital in 2016–2020. Neoadjuvant therapy was administered to only 4% of PDAC patients in 2011–2015, but to 20% in 2016–2020.
Discussion
Our retrospective cohort study on 566 pancreatectomies showed low postoperative mortality (30- and 90-day), and below-benchmark levels (6,7,35) of complications. Short-term outcomes remained mostly constant over twenty years, but 90-day mortality decreased from 3.1% to 0.74%, while the rate of postoperative interventions increased in the latest time quartiles. Overall 5-year survival improved over the twenty-year study period, but with no statistical significance. The resection rate increased substantially from 3.2% to 14.9%, thus reflecting a major improvement in the access to treatment rate.
The strength of our study is in the detailed data collection in a single-institution-setting. The cohort is complete with respect to short-term results, and survival follow-up. The data were collected by surgeons specialized in pancreatic surgery, and thus a biased assessment of complications, staging, or surgical techniques is unlikely. Still, incomplete information regarding the actual administering of adjuvant therapy for patients treated at secondary hospitals remains a weakness in the data collection. However, since the adjuvant treatment protocols are generally congruent between tertiary and secondary hospitals in Finland, we can assume that adjuvant therapy rates at Oulu University Hospital apply to the entire cohort. Insufficient reporting of pancreatic specimens is another weakness. The PD specimens are known to be challenging for pathologists. Especially the lymph node yield and margin status depend significantly on specimen processing. A structured pathology reporting method according to the Leeds Protocol (36) was not adopted until 2020 at Oulu University Hospital. Thus, positive surgical margins, tumor size, and invasion characteristics are probably underreported, especially in the earlier time periods.
We hypothesized, that the advances in complication management, emergence of adjuvant treatment, and centralization of pancreatic surgery would affect short- and long-term results in our cohort.
Complication management has improved due to advances in perioperative treatment and interventional radiology (5). This development reflects on the increased rate of percutaneous drainages and endovascular procedures in the last two time periods, and possibly in the lower rate of 90-day mortality (3.1% in 2000–2010 vs. 0.74% in 2011–2020). Reoperation rate increased significantly to 16.2% during the last decade, but the rate seems acceptable compared to the only benchmark study available by Sabater et al., who defined the acceptable quality limit for reoperation rate at <20%, with weighted average of 11% (35). Single center reports present slightly lower figures of 5–13% (37,38). Increment in reoperation rate may also indicate a prompter approach to addressing complications, supported by the corresponding decrease in 90-day mortality. The highly conservative approach to septic fistula complications in our center probably also influences the observed trend in reoperations—no completion pancreatectomies have been performed during the 20-year study period. Instead, the most common indication for relaparotomy was indeed fascial dehiscence, which may reflect the poorer performance status of the patients in the later time periods. The substantial rise in the resection rate in the last two time periods also relates to the reoperation rate. Increased number of resected stage III tumors, higher rate of vascular resections, and higher frequency of ASA III patients in the last time periods all support extension of operative indications to higher risk patients and procedures. Longer duration of surgeries in the last time periods is similarly most likely due to more advanced procedures. Higher risk, by definition, includes operating on patients with more advanced disease, more technically demanding cases, and patients with poorer preoperative performance status—all features associated with higher reoperation rate (37).
Resected PDAC patients [240] were separately analyzed for long-term results. The OS showed slight improvement when analyzed by time quartiles. The overall 3-year survival increased from 31.4% in 2006–2011 to 39.4% in 2016–2020, and the 5-year survival showed an improving trend from 14.3% in 2006–2011 to 21.4% in 2011–2015. The current survival rates are congruent with outcomes of high-volume pancreatic surgery centers (16,39). This conclusion is supported by the meticulous composition of our PDAC cohort, of which we excluded the less aggressive IPMN-, and MCN- originated adenocarcinomas (40). The trend of improving survival is more evident, if considered in the light of resection rate. Resection rate of PDAC has been reported to show large international variations, ranging from 13% to 21% in Europe and USA (41). In a retrospective, nationwide registry-based study, Aaltonen et al. found an 8% resection rate across Finnish centers (15). In our cohort, the three early quartiles carried a very low resection rate, increasing slowly from 3.2% to 5.9% in 2000–2015. However, in 2016–2020, the resection rate was substantially better (14.9%), while survival remained comparable (39.4% 3-year OS). Thus, a significantly larger number of pancreatic cancer patients were reached for curative intent surgery and multidisciplinary therapies in the last quartile. This evolution can be mainly attributed to the implementation of a specialized hepatobiliary and pancreatic MDT at Oulu University Hospital in 2015, adopting the BRPC and neoadjuvant therapy concepts to practice, national and international trends of PDAC surgery centralization, and oncological advancements.
An adjuvant treatment regimen with single gemcitabine was launched in 2004 at Oulu University Hospital. This does not however directly influence survival results—most likely due to the small cohort size—which did not seem to improve until 2011. The dual therapy with gemcitabine and capecitabine or FOLFIRINOX emerged after year 2017, and thus may only have impacted on the survival in the last quartile. In the last two quartiles, the adjuvant therapy rate was 80%, which is excellent compared to previous multicenter studies reporting adjuvant therapy rates of 58–65% (6,42). Neoadjuvant therapy for BRPC was properly launched in 2016 at our hospital. During the last quartile, neoadjuvant therapy was employed in 96% of resected BRPC patients, suggesting good compliance with guidelines.
Northern Finland pancreatectomies were centralized in 2011, when all four regional secondary hospitals jointly decided to refer pancreatic surgery to Oulu University Hospital. Centralization readily led to an increase in the number of pancreatic operations in years 2011–2015 compared to the previous time period 2006–2010 (n=96 vs. 139). Respectively, an increase in the 5-year OS (14.3% vs. 21.4%) was observed. The globally growing incidence of PDAC (43), and further the improved resection rate in our center, nearly doubled the number of pancreatic resections (n=266) in 2016–2020, with a corresponding rise in the 3-year OS compared to 2006–2010 (31.4% vs. 39.4%). The yearly volume of over 30–40 pancreatic resections has been suggested as a cut-off correlating with improved short- and long-term results (2,16). In line with this, our report shows that the current hospital volume of pancreatic surgery in our center is sufficient for maintaining the high quality of the operative process and for producing internationally comparable long-term outcomes.
Conclusions
We describe a substantial increase in the hospital-volume of pancreatic resections at Oulu University Hospital during 2000–2020, evaluated in four time periods. Based on assessment of our short-term outcomes, pancreatic surgery has been performed with a below-benchmark level of complications, and very low 90-day mortality. Despite the extension of operative indications to more advanced disease, more complex surgeries, and patients with poorer performance status, the overall 5-year survival of PDAC patients has improved to 21.4% over the study period, with a simultaneous rise in the resection rate from 3.2% to 14.9%.
Acknowledgments
Funding: This work was supported by grants from The Finnish Cultural Foundation (to HH), Vieno and Alli Suorsa Healthcare Foundation (to HH), Georg C. and Mary Ehrnrooth Foundation, Finnish State Research Fund (to HH) and Finnish Medical Foundation (to HH).
Footnote
Reporting Checklist: The authors have completed the STROCSS reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-649/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-649/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-649/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-649/coif). HH reports funding from The Finnish Cultural Foundation, Vieno and Alli Suorsa Healthcare Foundation, Georg C. and Mary Ehrnrooth Foundation, Finnish State Research Fund, and Finnish Medical Foundation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study complied with the ethical standards of Oulu University institutional research committee and the Declaration of Helsinki (as revised in 2013). The data collection from Finnish Cancer Registry was approved by the Finnish Social and Health Data Permit Authority (FINDATA, Dnro THL/3606/14.02.00/2020). The data collection was accepted by the Oulu University Hospital Ethics Committee (EETTMK: 81/2008) and by the National Supervisory Authority for Welfare and Health (VALVIRA; approval No. 10832/06.01.03.01/2014). Individual patient consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lermite E, Sommacale D, Piardi T, et al. Complications after pancreatic resection: diagnosis, prevention and management. Clin Res Hepatol Gastroenterol 2013;37:230-9. [Crossref] [PubMed]
- Hata T, Motoi F, Ishida M, et al. Effect of Hospital Volume on Surgical Outcomes After Pancreaticoduodenectomy: A Systematic Review and Meta-analysis. Ann Surg 2016;263:664-72. [Crossref] [PubMed]
- Ahola R, Siiki A, Vasama K, et al. Effect of centralization on long-term survival after resection of pancreatic ductal adenocarcinoma. Br J Surg 2017;104:1532-8. [Crossref] [PubMed]
- Nießen A, Hackert T. State-of-the-art surgery for pancreatic cancer. Langenbecks Arch Surg 2022;407:443-50. [Crossref] [PubMed]
- Baker TA, Aaron JM, Borge M, et al. Role of interventional radiology in the management of complications after pancreaticoduodenectomy. Am J Surg 2008;195:386-90; discussion 390. [Crossref] [PubMed]
- Sánchez-Velázquez P, Muller X, Malleo G, et al. Benchmarks in Pancreatic Surgery: A Novel Tool for Unbiased Outcome Comparisons. Ann Surg 2019;270:211-8. [Crossref] [PubMed]
- Ou C, Rektorysova M, Othman B, et al. Benchmarking Performance in Pancreatic Surgery: a Systematic Review of Published Quality Metrics. J Gastrointest Surg 2021;25:834-42. [Crossref] [PubMed]
- Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol 2019;20:1493-505. [Crossref] [PubMed]
- Finnish Cancer Registry. Cancer in Finland 2019. Annual report on cancer in Finland - Syöpärekisteri (cancerregistry.fi). 2019. Available online: http://syoparekisteri.fi/assets/files/2021/07/Cancer_in_Finland_2019.pdf
- Labori KJ, Katz MH, Tzeng CW, et al. Impact of early disease progression and surgical complications on adjuvant chemotherapy completion rates and survival in patients undergoing the surgery first approach for resectable pancreatic ductal adenocarcinoma - A population-based cohort study. Acta Oncol 2016;55:265-77. [Crossref] [PubMed]
- Picozzi VJ, Oh SY, Edwards A, et al. Five-Year Actual Overall Survival in Resected Pancreatic Cancer: A Contemporary Single-Institution Experience from a Multidisciplinary Perspective. Ann Surg Oncol 2017;24:1722-30. [Crossref] [PubMed]
- Seppänen H, Juuti A, Mustonen H, et al. The Results of Pancreatic Resections and Long-Term Survival for Pancreatic Ductal Adenocarcinoma: A Single-Institution Experience. Scand J Surg 2017;106:54-61. [Crossref] [PubMed]
- Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol 2012;19:169-75. [Crossref] [PubMed]
- Serrano PE, Cleary SP, Dhani N, et al. Improved long-term outcomes after resection of pancreatic adenocarcinoma: a comparison between two time periods. Ann Surg Oncol 2015;22:1160-7. [Crossref] [PubMed]
- Aaltonen P, Carpén O, Mustonen H, et al. Long-term nationwide trends in the treatment of and outcomes among pancreatic cancer patients. Eur J Surg Oncol 2022;48:1087-92. [Crossref] [PubMed]
- Huhta H, Nortunen M, Meriläinen S, et al. Hospital volume and outcomes of pancreatic cancer: a Finnish population-based nationwide study. HPB (Oxford) 2022;24:841-7. [Crossref] [PubMed]
- Huang L, Jansen L, Balavarca Y, et al. Stratified survival of resected and overall pancreatic cancer patients in Europe and the USA in the early twenty-first century: a large, international population-based study. BMC Med 2018;16:125. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v56-68. [Crossref] [PubMed]
- Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. J Natl Compr Canc Netw 2019;17:603-5. [Crossref] [PubMed]
- Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977-88. [Crossref] [PubMed]
- Sanjay P, Takaori K, Govil S, et al. 'Artery-first' approaches to pancreatoduodenectomy. Br J Surg 2012;99:1027-35. [Crossref] [PubMed]
- Sell NM, Pucci MJ, Gabale S, et al. The influence of transection site on the development of pancreatic fistula in patients undergoing distal pancreatectomy: A review of 294 consecutive cases. Surgery 2015;157:1080-7. [Crossref] [PubMed]
- Pannageon V, Pessaux P, Sauvanet A, et al. Pancreatic Fistula After Distal Pancreatectomy: Predictive Risk Factors and Value of Conservative Treatment. Arch Surg 2006;141:1071-6. [Crossref] [PubMed]
- Agha R, Abdall-Razak A, Crossley E, et al. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg 2019;72:156-65. [Crossref] [PubMed]
- Armitage JN, van der Meulen JH. Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br J Surg 2010;97:772-81. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Besselink MG, van Rijssen LB, Bassi C, et al. Definition and classification of chyle leak after pancreatic operation: A consensus statement by the International Study Group on Pancreatic Surgery. Surgery 2017;161:365-72. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- WHO Classification of Tumours Editorial Board 2019, editor. WHO Classification of Tumours. Digestive System Tumours. 5th ed. Vol. 1. World Health Organization, 2019.
- Sabater L, Mora I, Gámez Del Castillo JM, et al. Outcome quality standards in pancreatic oncologic surgery in Spain. Cir Esp 2018;96:342-51. (Engl Ed). [Crossref] [PubMed]
- Verbeke CS, Leitch D, Menon KV, et al. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232-7. [Crossref] [PubMed]
- Lyu HG, Sharma G, Brovman E, et al. Risk Factors of Reoperation After Pancreatic Resection. Dig Dis Sci 2017;62:1666-75. [Crossref] [PubMed]
- Dusch N, Lietzmann A, Barthels F, et al. International Study Group of Pancreatic Surgery Definitions for Postpancreatectomy Complications: Applicability at a High-Volume Center. Scand J Surg 2017;106:216-23. [Crossref] [PubMed]
- Girgis MD, Zenati MS, King JC, et al. Oncologic Outcomes After Robotic Pancreatic Resections Are Not Inferior to Open Surgery. Ann Surg 2021;274:e262-8. [Crossref] [PubMed]
- Aronsson L, Bengtsson A, Torén W, et al. Intraductal papillary mucinous carcinoma versus pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Int J Surg 2019;71:91-9. [Crossref] [PubMed]
- Huang L, Jansen L, Balavarca Y, et al. Resection of pancreatic cancer in Europe and USA: an international large-scale study highlighting large variations. Gut 2019;68:130-9. [Crossref] [PubMed]
- Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260:372-7. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]